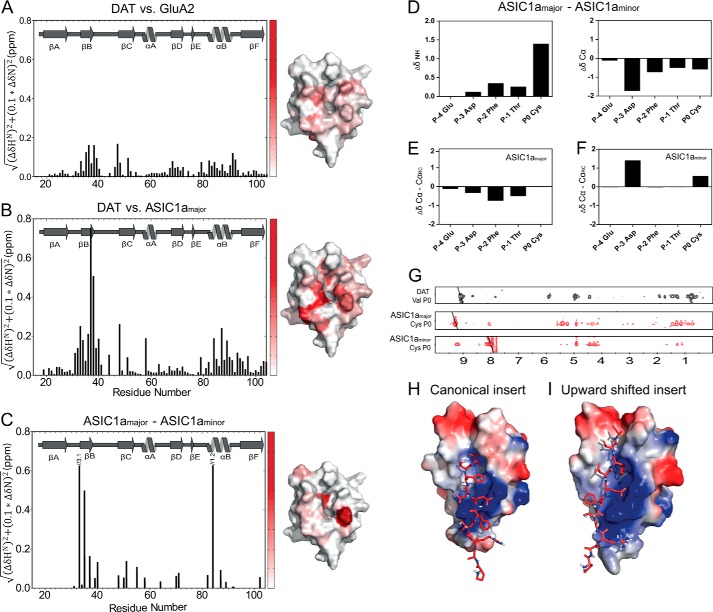
FIGURE 4.
Ligand-dependent differences in chemical shifts for the PICK1 PDZ domain. The histograms show weighted differences in backbone NH chemical shifts between the DAT construct and the GluA2 construct (A), and the DAT construct and the ASIC1a construct (B), and ASIC1a major and minor state (C). All spectra were recorded at 15 °C using the same experimental settings. The differences in backbone NH chemical shifts between the five C-terminal ASIC residues in the major and the minor state are shown in D, as well as a secondary chemical shift analysis of each state in E and F. 15N-filtered NOEs obtained for the Cys at P0 in ASIC1a in both states are compared with corresponding NOEs obtained for Val in the DAT C terminus used for structural determination (G). Docking models of the ASIC1a C11 peptide onto the PICK1-PDZ domain based on the chemical shift differences in B and C yielded two clusters using the HADDOCK protocol suggesting that the ASIC1a peptide adopts two different binding conformations as follows: a class II-like insertion (H) and an insertion where the ligand is shifted two residues upward (I). ASIC C11 is shown as red sticks, and the PDZ domain is colored with the electrostatic potential from −2 kT/e (blue) to 2 kT/e (red).