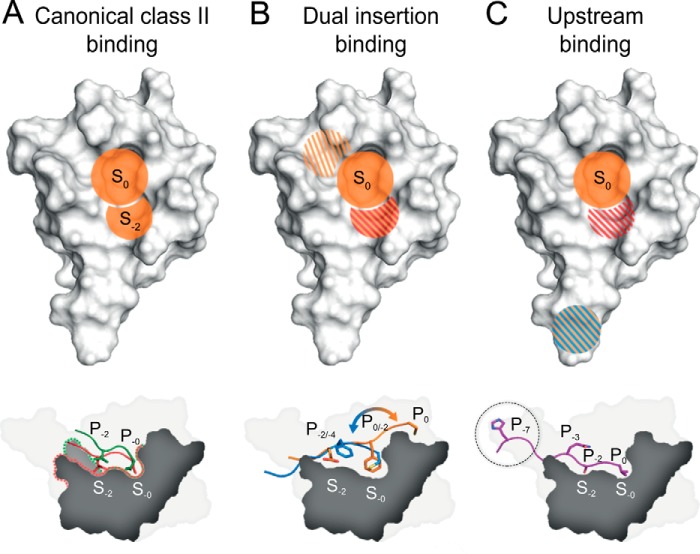
FIGURE 6.
Binding modes of the PICK1 PDZ domain. A, the PICK1 PDZ domain has the highest affinity for class II ligands that is primarily mediated by hydrophobic pocket S0 and S−2 (orange regions). Both patches contribute equally to the affinity. B, class II-like ligands containing a C-terminal Cys may bind similarly to regular class II ligands with the Cys binding in hydrophobic pocket S0. However, we also observed an alternative binding mode in which the hydrophobic P−2 residue binds in hydrophobic pocket S0 and with either hydrophobic or negatively charged residues (shaded red/orange) mimicking in the hydrophobic pocket S−2. The additional upper binding site, situated outside the defined PDZ binding groove and binding the residues in P1 and P0 of the ligand, is slightly hydrophobic (shaded orange). C, class I ligands make hydrophobic contacts in the hydrophobic pocket S0 (orange); Lys-83 contributes to an electrostatic favorable environment for Ser/Thr residues at P−2 of class I ligands (shaded red). Furthermore, an additional upstream binding site preferring positively charged and/or bulky hydrophobic residues resides in the flexible loop region (shaded blue/orange).