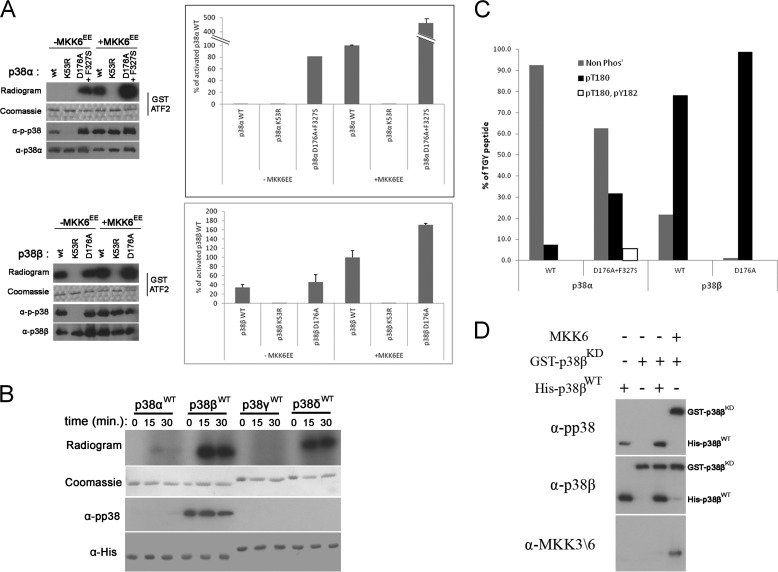
FIGURE 1.
p38β, but not p38α, is intrinsically active. A, in vitro kinase assay was preformed with the indicated p38 proteins with GST-ATF2 as a substrate. Activity is shown in graphs, expressed as percentages of the activity of MKK6-activated wild type p38α or p38β (100%), and by autoradiograms. 100 ng of each protein was also analyzed by Western blot with the indicated antibodies. B, in vitro autophosphorylation assay was preformed with the indicated p38 isoforms. Activity is shown by autoradiograms. 100 ng of each protein was also analyzed by Western blot with the indicated antibody. C, 5 μg of p38αWT, p38αD761A+F327S, p38βWT, and p38βD176A were incubated with ATP for 1 h in a kinase reaction mixture with no other substrate (autophosphorylation). Proteins were then cleaved into tryptic peptides and analyzed for phosphorylation sites by tandem MS. The relative intensities of the TGY motif-containing peptides in the different phosphorylation states: nonphosphorylated (Non Phos), Thr180-phosphorylated, and Thr180 + Tyr182-phosphorylated (0P, 1P, and 2P) are displayed as the percentages of all TGY peptides intensities (0P + 1P + 2P). D, 250 nm of purified His-tagged p38βWT or of MKK6EE were incubated with 250 nm GST-tagged p38βKD in a kinase reaction mixture for 1 h. Samples of each reaction were analyzed by Western blot with the indicated antibodies.