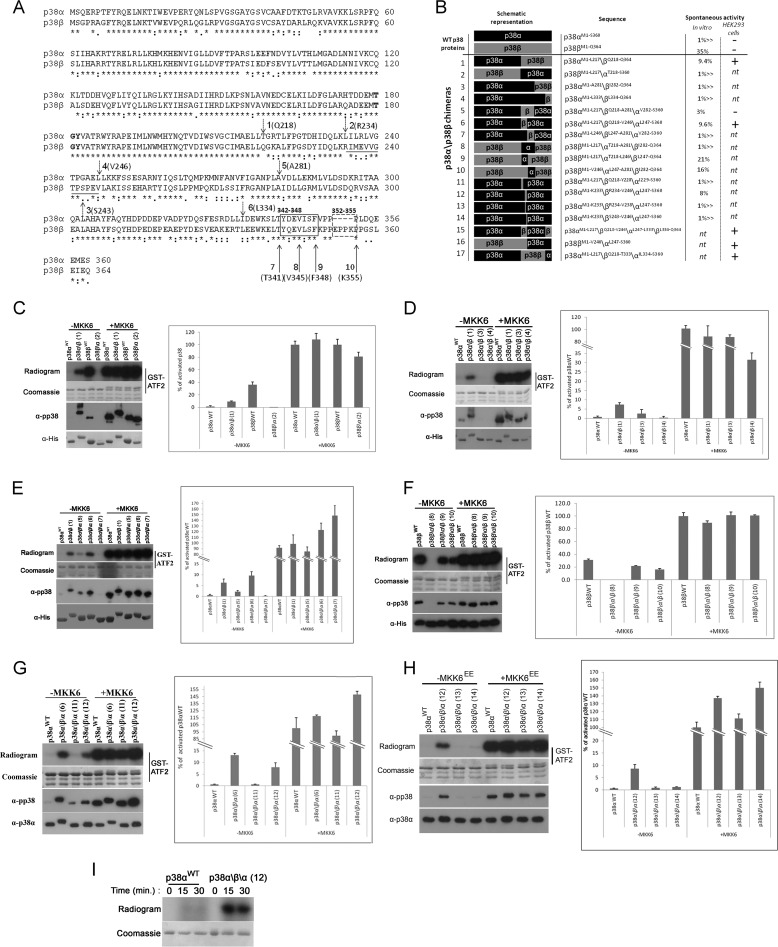
FIGURE 2.
A 13-amino acid region, Arg234–Val246, which composes part of the G-helix and the MKI of p38β, is responsible for its autophosphorylation activity. A, p38α and p38β linear sequences were aligned using the ClustalW server. Bold type, TGY motif; dashed arrows 1–6, chimera swap points; black line under alignment, Arg234–Val246 region; solid arrows 7–10, truncation sites; solid and dashed boxes, Tyr342–Phe348 and Glu352–Lys355 regions. B, summary of the chimeras used in this study, showing their measured activity in in vitro kinase assays as percentages of the activity of MKK6-activated p38 and their activity in HEK293 cells. C–H, results of kinase assays and Western blots preformed with p38α\p38β chimeras. The results are shown in gels and graphs as described in legend of Fig. 1. p38 chimeras are numbered according to B. C, activities of chimeras 1 and 2 compared with those of MKK6-activated p38αWT and p38βWT. D, activities of chimeras 1, 3, and 4 compared with MKK6-activated p38αWT. E, activities of chimeras 1, 5, and 6 compared with MKK6-activated p38αWT. F, activities of chimeras 8, 9, and 10 compared with MKK6-activated p38βWT. G, activities of chimeras 6, 11, and 13 compared with MKK6-activated p38βWT. H, activities of chimeras 12, 13, and 14 compared with MKK6-activated p38αWT. I, p38αWT and p38α\β\α chimera 12 were tested in an autophosphorylation assay. Samples were collected at the indicated times, separated by SDS-PAGE, and exposed to film.