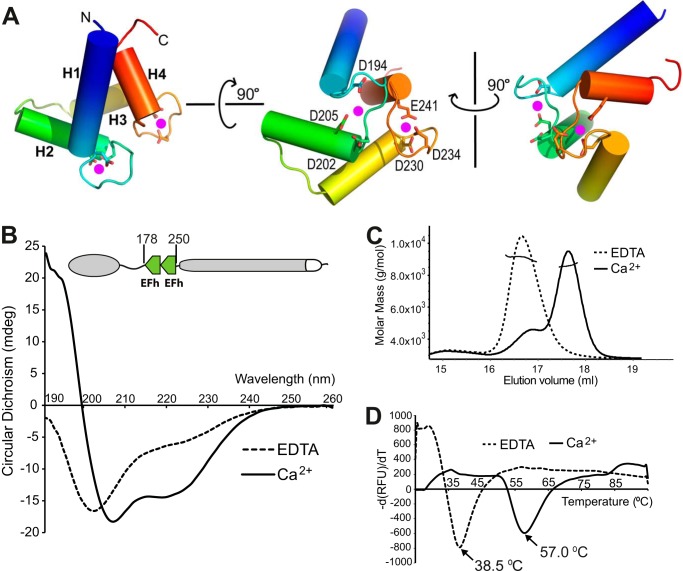
FIGURE 3.
Calcium binding induces structural changes in the TbBILBO1-EFh motifs. A, three orthogonal views of the TbBILBO1-EFh (residues 178–250) derived from homology-based modeling. The schematic is color-ramped from blue at the N terminus to red at the C terminus. The four α-helices (H1–H4) in the two EFh motifs are shown as cylinders. Conserved acidic residues in the loops, which are likely to coordinate calcium binding, are shown as sticks. The two predicted calcium-binding sites are shown as magenta dots. B, far-UV CD spectra of recombinant untagged TbBILBO1-EFh in the presence of either 2 mm CaCl2 or 2 mm EDTA. Complete removal of calcium by EDTA resulted in the apo-form of the protein with a classical random coil structure, showing a maximum below 0 millidegrees and a minimum at ∼200 nm. Conversely, calcium binding led to a characteristic α-helical spectrum with minima at 209 and 220 nm. C, SLS results of the two forms of the TbBILBO1-EFh. Although both were monomeric, the apo-form was eluted much earlier than the calcium-loaded form, suggesting that in the absence of calcium, the EFh is improperly folded and therefore more loosely packed. It therefore behaves like a larger protein. D, melting curves of the TbBILBO1-EFh with and without loaded calcium measured by ThermoFluor assays. The melting temperatures for the apo- and calcium-bound forms were 38.5 and 57.0 °C, respectively. This further demonstrates that binding to calcium makes the EFh undergo a conformational switch from unfolded random coils to a well folded compact structure, conferring greater thermal stability.