FIGURE 1.
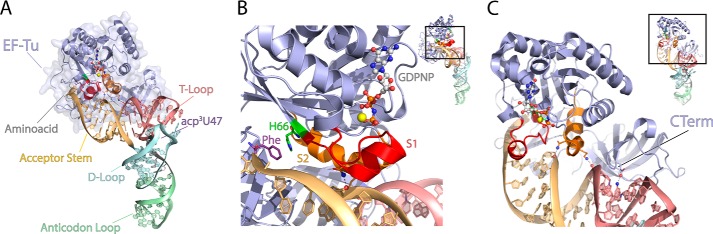
EF-Tu binds both the aminoacyl moiety and the tRNA body. A, E. coli EF-Tu (blue) bound in a ternary complex with GDPNP and Saccharomyces cerevisiae Phe-tRNAPhe (Protein Data Bank code 1OB2). The following functional domains of tRNA are highlighted: acceptor stem (orange), T-loop (red), D-loop (blue), and the anticodon loop (green) as well as GDPNP (carbon atoms in gray, nitrogen in blue, oxygen in red, and phosphorus in orange) and the acp3U47 residue, the site of fluorophore labeling used in this report. B, the aminoacyl binding pocket is stabilized by the GTP-dependent coordination of switch 1 (S1; red) and switch 2 (S2; orange) regions. C, conserved contacts between the acceptor stem of aa-tRNA and all three domains of EF-Tu. The approximate position of the C terminus (CTerm) of EF-Tu is shown.
