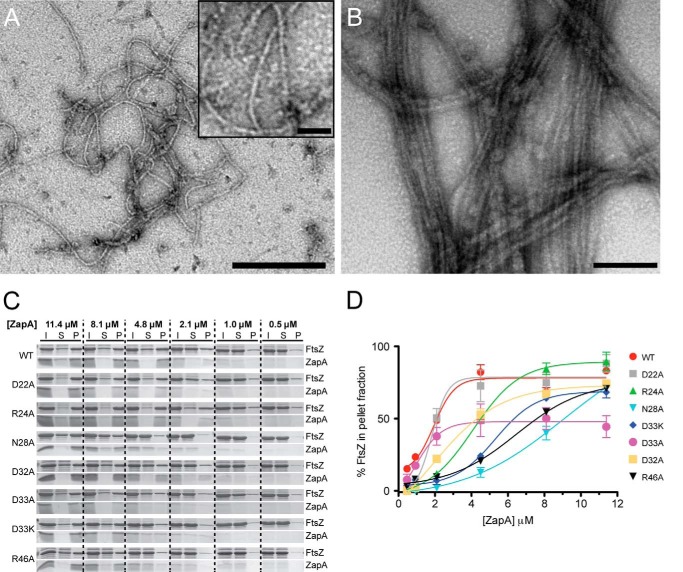
FIGURE 2.
FtsZ sedimentation assay and the percent of FtsZ in the pelleted fractions. A, purified FtsZ filamented with 1 mm GTP in the absence of ZapA (bar, 250 nm; inset bar, 25 nm). B, FtsZ filaments bundled with purified ZapA (4.8 μm; bar, 100 nm). C, FtsZ (4.8 μm) filamented with 1 mm GTP in the presence of WT ZapA or ZapA variants (0.5–11.4 μm). Initial samples (I) were used to assess the total protein content in each reaction. Soluble (S) and pelleted (P) fractions were separated by centrifugation at 10,000 rpm for 15 min, and all three samples (I, S, and P) were analyzed by Coomassie-stained SDS-PAGE. Pelleted fractions contain FtsZ filaments that were bundled with ZapA. D, triplicate FtsZ sedimentation assays were performed for each ZapA variant, and Coomassie-stained SDS-PAGE results were analyzed by densitometry. The mean percentages of FtsZ in the pelleted fractions were plotted for each concentration of ZapA, where the error bars indicate ± S.E. (standard error of the mean). Reactions with equimolar ZapA (4.8 μm) and FtsZ (4.8 μm), lacking GTP, were run as a control for protein precipitation (not shown), and these values were subtracted from the sedimentation of each reaction.