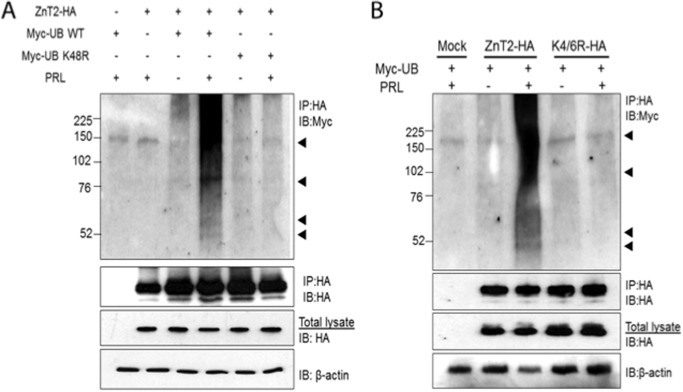
FIGURE 2.
PRL stimulated ZnT2 ubiquitination. A, cells were transfected to express ZnT2-HA and Myc-ubiquitin (Myc-UB WT) alone or in combination or to co-express ZnT2-HA and Myc-ubiquitin K48R (Myc-UB K48R). Following a 24-h recovery, cells were pretreated with CHX to inhibit protein synthesis and with MG132 to inhibit proteasomal degradation and then treated for an additional 8 h in medium (−) or medium containing PRL (+). Cell lysates were passed over anti-HA affinity matrix (IP:HA), and the retained fraction was separated by 4–20% SDS-polyacrylamide gradient gels. Membranes were immunoblotted with anti-Myc antibody (IP:HA and IB:Myc) and stripped and reprobed for anti-HA antibody (IP:HA and IB:HA) as a loading control. Total cell lysates from the same samples were used to detect total abundance of ZnT2-HA protein and β-actin. Experiments were repeated more than three times. B, cells were transfected to express ZnT2-HA and Myc-ubiquitin (Myc-UB) alone or in combination or to co-express ZnT2-HA and K4R/K6R-HA. Following a 24-h recovery, cells were pretreated with CHX to inhibit protein synthesis and with MG132 to inhibit proteasomal degradation and then treated for an additional 8 h in medium (−) or medium containing PRL (+). Cell lysates were passed over anti-HA affinity matrix (IP:HA), and the retained fraction was separated by 4–20% SDS-polyacrylamide gradient gels. Membranes were immunoblotted with anti-Myc antibody (IP:HA and IB:Myc) and stripped and reprobed for anti-HA antibody (IP:HA and IB:HA) as a loading control. Total cell lysates from the same samples were used to detect total abundance of ZnT2-HA protein and β-actin. Experiments were repeated more than three times.