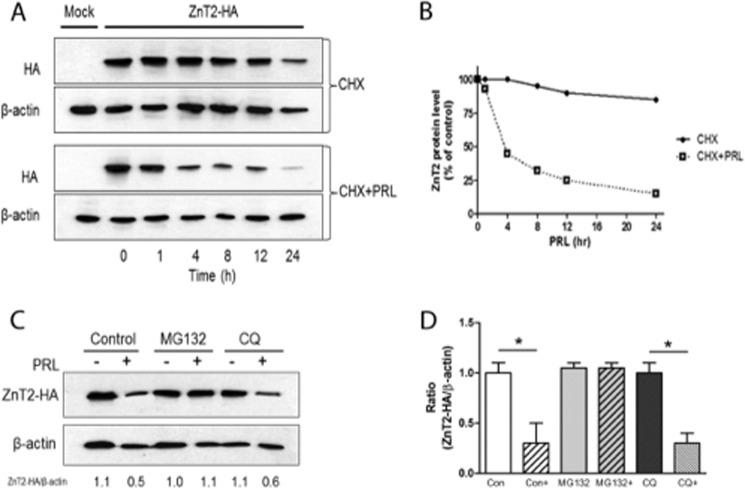
FIGURE 4.
PRL induced degradation of ZnT2. A, representative immunoblot of ZnT2-HA detected in the total membrane fraction of cells transfected with pcDNA3.1 (Mock) or cells expressing ZnT2-HA. Cells were treated with cycloheximide (100 μg/ml) alone (CHX) to inhibit new protein synthesis or in combination with PRL (CHX + PRL) for the indicated times. Total membrane fractions from the cells were separated by SDS-PAGE, and membranes were immunoblotted with HA antibody and then stripped and reprobed for β-actin as a loading control. B, ZnT2 protein abundance in response to PRL stimulation. Band intensity of ZnT2-HA was normalized to β-actin and expressed as a percentage of untreated cells. Data represent the mean percentage of ZnT2 protein level at time 0 ± S.D.; the experiment was repeated twice. C, representative immunoblot of ZnT2-HA detected in the total membrane fraction of cells transfected with ZnT2-HA. Cells were pretreated with cycloheximide (100 μg/ml) in the presence of MG132 (proteasome inhibitor) or chloroquine (CQ; lysosome inhibitor). Cells were then treated for an additional 8 h in medium (−) or medium containing PRL (+) in the presence of the above inhibitors. Total membrane fractions from the cells were separated by SDS-PAGE, and membranes were immunoblotted with anti-HA antibody and stripped and reprobed for β-actin as a loading control. Experiments were repeated more than three times. D, data represent the mean ratio of ZnT2/β-actin ± S.E. (error bars) from untreated cells (Con), untreated cells stimulated with PRL (Con+), cells pretreated with MG132 and stimulated with PRL (MG132+), or cells pretreated with chloroquine (CQ) and stimulated with PRL (CQ+) from two independent experiments. *, significant effect of PRL treatment on the ratio of ZnT2/β-actin, p < 0.05.