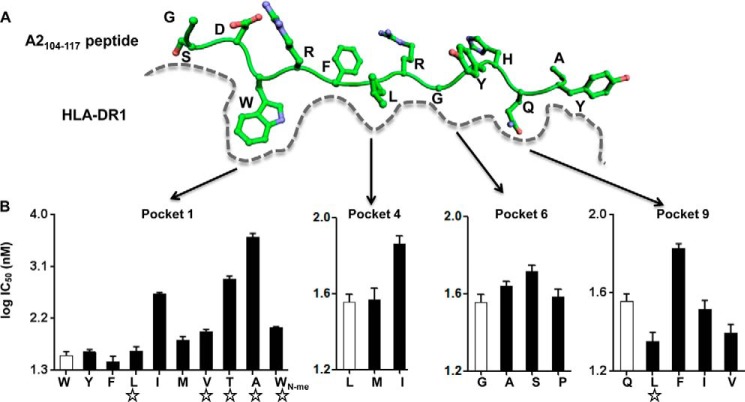
FIGURE 1.
Identification of peptides with weakened pocket 1 interactions and strengthened interactions at other pockets. A, schematic view of peptide binding groove from previously solved crystal structure of peptide HLA-A2(104–117) bound to DR1 (PDB code 1AQD). Pockets 1, 4, 6, and 9 on DR1, which harbor the major anchor residues of HLA-A2(104–117), are indicated. B, competition binding with indicator peptide Alexa488-HA(306–318) to DR1 was measured for HLA-A2(104–117)-derived peptides harboring single mutation at pockets 1, 4, 6, and 9 anchor residues. The amino acid after substitution for each peptide was labeled at the x axis, with the open bar indicating wild-type sequence, and the star indicating peptides selected for following detailed studies. These data represent three independent experiments with two replicates each.