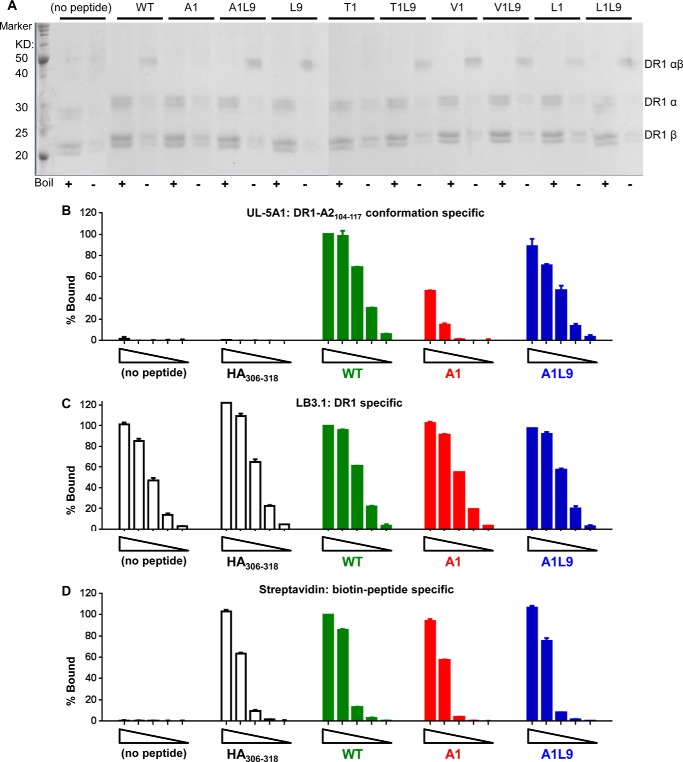
FIGURE 6.
DR1-A1 shows SDS-sensitive conformation and decreased recognition by a conformation-specific monoclonal antibody UL-5A1. A, SDS stability of empty DR1 or DR1 loaded with different peptides was determined. Each sample was split into two with one boiled (+) and the other one not (−). A protein marker was included in the 1st lane. The bands for α-subunit, β-subunit, and αβ complex were indicated. The multiple bands for α-subunit and β-subunit were due to glycosylation of DR1 produced in insect cells, as described previously (75). This gel is representative of three independent experiments. Detection of empty DR1, DR1 bound with HA(306–318), WT, A1, and A1L9 by UL-5A1 (B), LB3.1 (C), and streptavidin (D) was shown after BSA background subtraction. Each complex started at 500 ng with a diluting factor of 5. Each peptide was labeled with biotin. Percent bound (% Bound) was normalized to the absorbance of 500 ng of DR1-WT. Data are representative of at least two independent experiments with two replicates each.