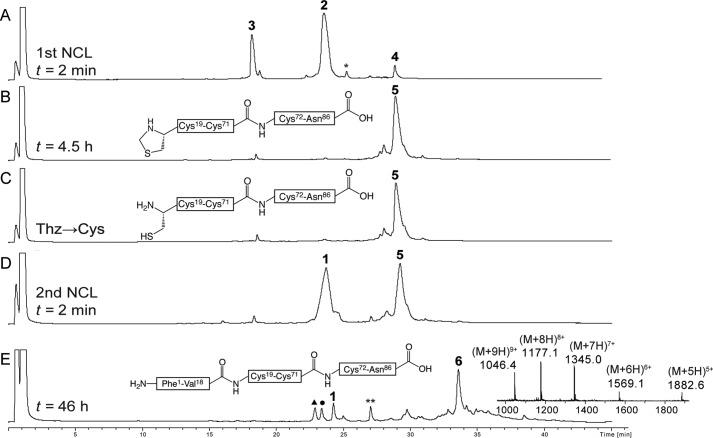
FIGURE 3.
Analytical LC-MS data for one pot total chemical synthesis of [d-AlaB8]proinsulin. A, native chemical ligation of Cys72-Asn86 (3) and Thz19-Cys71-αCOSR (2) (r = -CH2CH2CO-Ala-COOH) to give Thz19-Asn86 (4) at t = 2 min. The asterisk (*) corresponds to the MPAA-exchanged thioester intermediate form of 2. B, the same ligation reaction upon completion after 4.5 h. C, crude reaction mixture after treatment with methoxylamine/HCl at pH 4.0 to convert Thz to Cys. D, native chemical ligation of Cys19-Asn86 (5) and [d-AlaB8]Phe1-Val18-αCOSR (1) to give the full-length polypeptide [l-AlaB8]Phe1-Asn86 (6) at t = 1 min. E, the same ligation reaction upon completion after 46 h. Because peptide segment 1 was used in excess (1.5 eq), several related peaks were observed at the end of the second native chemical ligation: (1) unreacted peptide segment; **, MPAA-exchanged thioester intermediate form of 1; ▴, hydrolysis of the thioester of peptide 1; ●, product of reaction of methoxylamine with thioester 1. Online ESI MS data (inset) is shown (observed mass, 9408.4 ± 0.3 Da; calculated mass (average isotope composition), 9408.6 Da). Analytical HPLC (λ = 214 nm) was performed using a linear gradient (5–47%) of buffer B in buffer A over 42 min (buffer A = 0.1% TFA in water; buffer B = 0.08% TFA in acetonitrile) on a C18 column.