FIGURE 1.
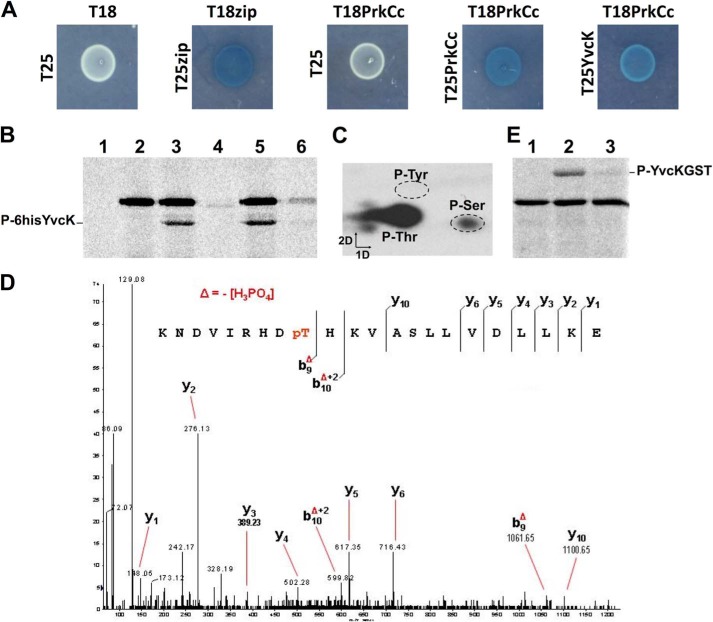
Phosphorylation of YvcK by PrkC at Thr-304. A, interaction between PrkC and YvcK by bacterial two-hybrid assay. The T18 and T25 fragments of the adenylate cyclase protein were fused to the N termini of PrkCc and YvcK. Cotransformed strains of E. coli BTH101 were spotted onto LB medium supplemented with X-Gal and IPTG and incubated overnight at 30 °C. Blue colonies indicate a positive interaction of the two moieties of the β-galactosidase enzyme and hence a positive interaction of PrkCc and YvcK. B, phosphorylation of YvcK by PrkCc. The YvcK protein was expressed either with a C-terminal His6 tag (A–C) yielding an ∼35-kDa protein, or with an N-terminal GST tag (D), yielding a 60-kDa protein. The recombinant proteins were purified and then incubated with [γ-33P]ATP and PrkCc. Myelin basic protein and [γ-33P]ATP were incubated at 37 °C for 15 min in the presence of YvcK alone (lane 1), in the presence of PrkCc alone (lane 2), in the presence of PrkCc and YvcK (lane 3), in the presence of PrkCc K40A and YvcK (lane 4), or in the presence of PrkCc and YvcK with the addition of either buffer (lane 5) or PrpC (lane 6) after 7.5 min of incubation. Samples were separated by SDS-PAGE and visualized by autoradiography. C, phosphoamino acid determination. Phosphorylated YvcK was purified and hydrolyzed, and the phosphorylated amino acid residues were separated by electrophoresis in the first dimension and by ascending chromatography in the second dimension. The positions of the unlabeled phosphoamino acid standards are circled. D, identification of Thr-304 as the phosphorylated residue in YvcK by mass spectrometry. Shown is the MS/MS spectrum of the doubly charged ion [M + 2H]2+ at m/z 656.6 of peptide-(296–317) (monoisotopic mass of 2622.42 Da). The phosphate group on Thr-304 was identified by observation of the y C-terminal daughter ion series. Starting from the C-terminal residue, all y ions lose phosphoric acid (−98 Da) after the phosphorylated Thr-304 residue. E, phosphorylation of the GST-YvcK T304A mutant. Two micrograms of purified GST (lane 1), GST-YvcK (lane 2), and GST-YvcK T304A (lane 3) were incubated with myelin basic protein, PrkCc, and [γ-33]ATP. Samples were separated by SDS-PAGE and visualized by autoradiography.