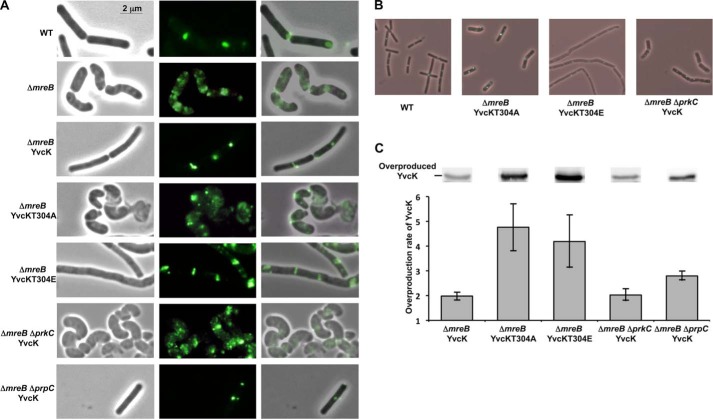
FIGURE 4.
Impact of phosphorylation on the ability of YvcK to rescue an mreB mutant. A, cell shape and cellular localization of GFP-PBP1 in mreB mutant strains upon YvcK overproduction. Strains were grown on LB medium, and YvcK proteins were expressed from the inducible Pspac promoter in the presence of 100 μm IPTG, whereas the gfp-ponA gene fusion was expressed from the inducible Pxyl promoter in the presence of 0.5% xylose. The cell shape (left panels) and PBP1 localization (middle panels) were analyzed by microscopy for strains YK706 (WT), SG204 (ΔmreB), SG217 (ΔmreB, pDG148-YvcK), SG262 (ΔmreB, pDG148-YvcK T304A), SG281 (ΔmreB, pDG148-YvcK T304E), SG265 (ΔmreB ΔprkC, pDG148-YvcK), and SG413 (ΔmreB ΔprpC, pDG148-YvcK). Merged images of phase and fluorescent micrographs are shown (right panels). B, of cell shape and intracellular localization of GFP-PBP1 upon either YvcK T304A or YvcK T304E overproduction in an mreB mutant strain. Strains were grown on LB medium supplemented with 25 mm MgSO4, and YvcK proteins were expressed from the inducible promoter in the presence of 100 μm IPTG, whereas the gfp-ponA gene fusion was expressed from the inducible promoter in the presence of 0.5% xylose. Cell shape and PBP1 localization were analyzed by microscopy, and an overlay of phase and fluorescent micrographs is presented for strains YK706 (WT), SG262 (ΔmreB, pDG148-YvcK T304A), SG281 (ΔmreB, pDG148-YvcK T304E), and SG265 (ΔmreB ΔprkC, pDG148-YvcK). C, analysis of YvcK overproduction by Western blotting. Strain SG204 (ΔmreB) and overproducing strains SG217 (ΔmreB, pDG148-YvcK), SG262 (ΔmreB, pDG148-YvcK T304A), SG281 (ΔmreB, pDG148-YvcK T304E), SG265 (ΔmreB ΔprkC, pDG148-YvcK), and SG413 (ΔmreB ΔprpC, pDG148-YvcK) were grown on Difco Antibiotic Medium 3 (PAB) medium supplemented with 0.3 m sucrose and 25 mm MgSO4 to allow normal growth of mreB mutants (20) and with 100 μm IPTG until A600 = 1. After centrifugation, the pellets were resuspended in 0.1 volume of lysis buffer. For each, 8 and 16 μl of crude extract were separated by SDS-PAGE. After blotting, YvcK was detected using antibodies directed against YvcK as described previously (16). For each overproducing strain, the image shows the signal obtained for overproduced YvcK from 16 μl of crude extract. To estimate the relative quantity of YvcK in crude extract and to compare the different lanes and different blots (for each strain analyzed, 16 and 8 μl of crude extract were loaded on the gel), we used an internal standard, the YpmB protein, which was detected using specific antibodies (1:15,000 dilution). The level of YvcK overproduction is the ratio of YvcK quantities determined using ImageJ in an overproducing strain and in the non-overproducing strain SG204.