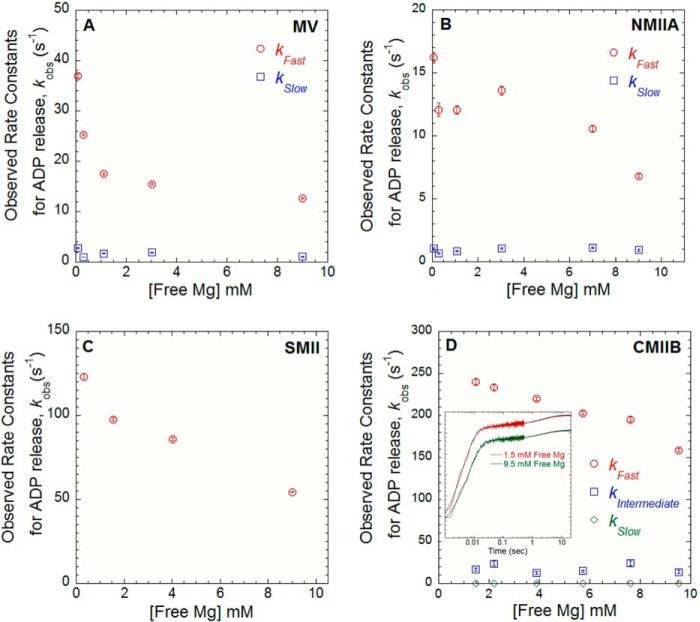
FIGURE 2.
Mg2+-dependent ADP release in MV, NMIIA, SMII, and CMIB. The ADP release rate constants were examined by mixing acto-MV (A), -NMIIA (B), and -SMII (C) in the presence of mant-dADP with 1 mm ATP. The free Mg2+ was varied between 0.3 and 9 mm at 25 °C (final reaction conditions: 0.5–0.75 μm myosin, 0.75–1 μm actin, 10–20 μm mant-dADP, and 1 mm ATP). D, ADP release rate constant was measured in CMBII by performing ATP-induced dissociation from pyrene actin in the presence of ADP. A complex of pyrene acto-CMIIB·ADP was mixed with ATP, and the resulting fluorescence transients (see inset) were best fit by a three-exponential function (final reaction conditions: 0.8 μm CMIIB and pyrene actin, 160 μm ADP, and 16 mm ATP). The observed rates of the fast, intermediate, and slow phases are plotted as a function of free Mg2+ concentration. The relative amplitudes were similar at all free Mg2+ concentrations measured (fast = 0.9, intermediate = 0.02, and slow = 0.08).