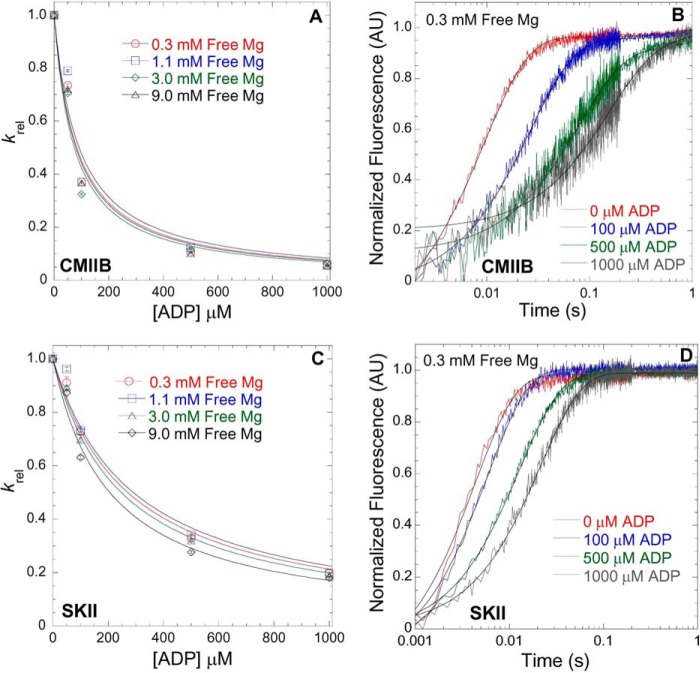
FIGURE 3.
Mg2+-dependent ADP affinity in CMIIB and SKII. ATP-induced dissociation from pyrene actin in the presence of ADP was utilized to measure the ADP affinity in CMIIB (A) and SKII (C) in the presence of 0.3, 1.1, 3.0, and 9.0 mm free Mg. The fluorescence (arbitrary units, AU) transients were fit to a single exponential, and the krel (krel = kobs/k0, where k0 = kobs in the absence of ADP) values were plotted as a function of [ADP]. The ADP affinity was determined from the hyperbolic fits (krel = 1/(1 + [ADP]/K′ADP, where K′ADP = the apparent affinity for ADP) at each free concentration as is shown for 0.3 mm free Mg in CMIIB (B) and SKII (D) (final reaction conditions: 0.5 μm myosin, 1 μm pyrene actin, 50 μm ATP, and varying concentrations of ADP).