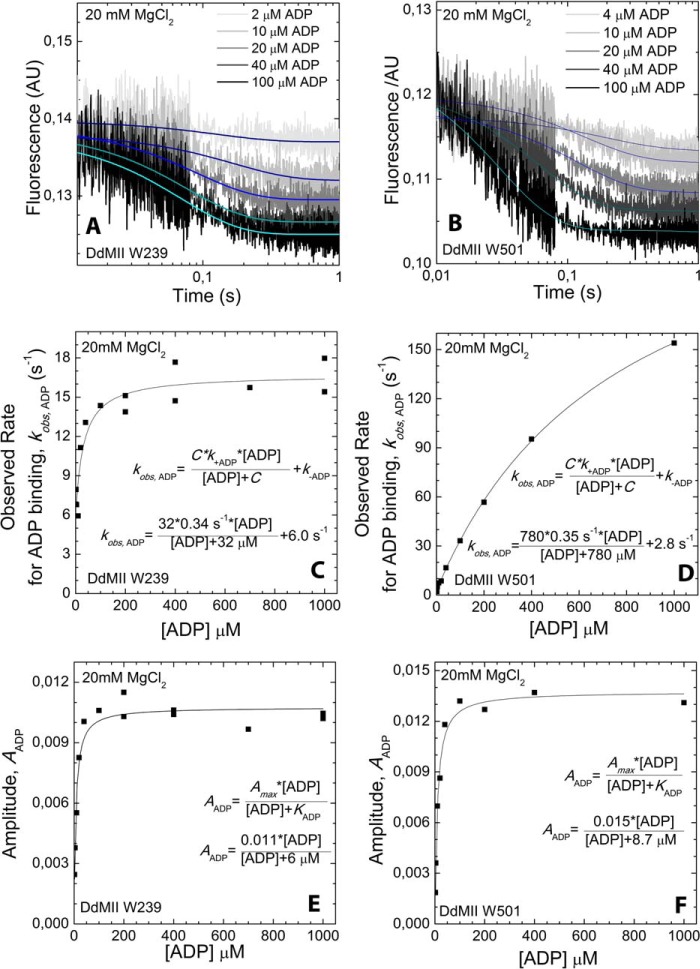
FIGURE 6.
ADP binding to DdMII motor domain in the presence of 20 mm MgCl2. Trp-239 DdMII (A) and Trp-501 DdMII (B) fluorescence (arbitrary units, AU) transients upon binding with increasing concentrations of ADP are shown. Mixing conditions are as follows: 3–5 μm DdMII was mixed with increasing concentrations of ADP; both syringes contained 20 mm HEPES, 60 mm NaCl, and 20 mm MgCl2, pH 7.3 buffer, at 13 °C. Single exponential decays were fitted to the averages of 3–5 measurements. ADP concentration dependence of the fitted observed rates is shown for Trp-239 DdMII (C) and Trp-501 DdMII (D). The ADP binding to the DdMII motor domain was treated kinetically as a second-order reaction. The observed rate constants fit a hyperbolic function of ADP concentration. The hyperbolic fit is indicated on each graph; C is the negative vertical asymptote, and k+ADP and k–ADP are the binding and unbinding rate constants; respectively. ADP concentration dependence of the fitted amplitudes of the fluorescent transients is also shown for Trp-239 DdMII (E) and Trp-501 DdMII (F). KADP, the ADP affinity of DdMII is calculated from the hyperbolic ADP concentration dependence of the amplitudes. The calculated rate constants and ADP affinities from C, D, E, and F are summarized in Table 2.