Figure 1. GSNO production from nanoparticles.
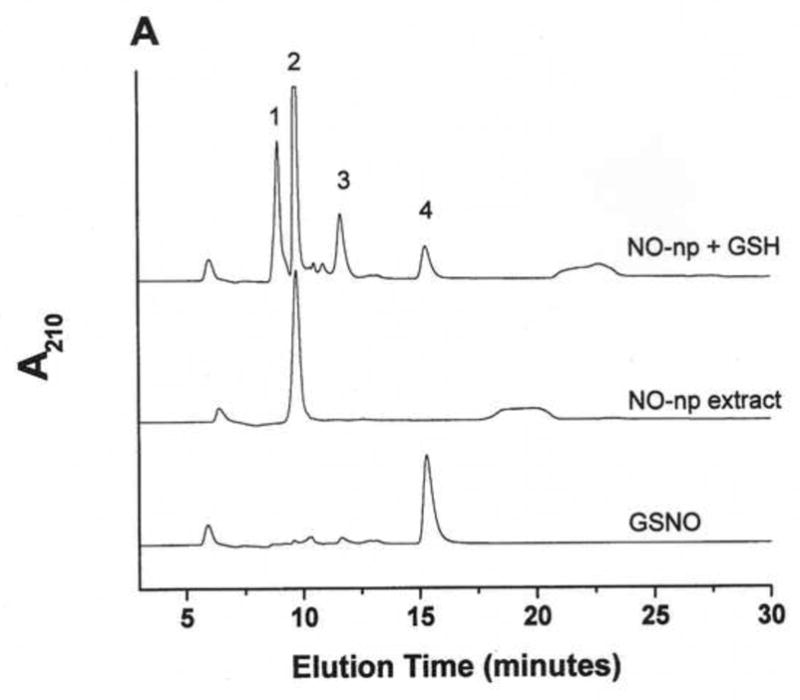
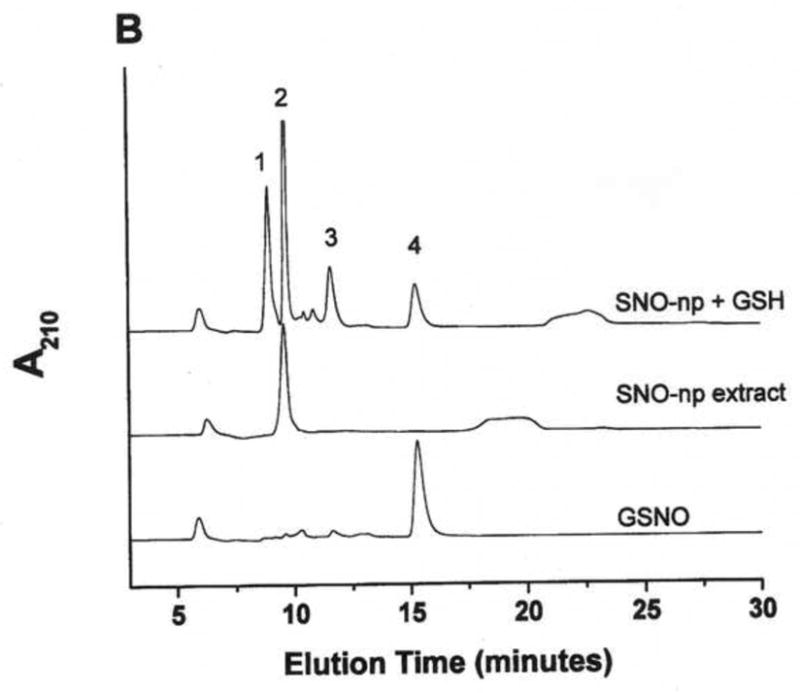
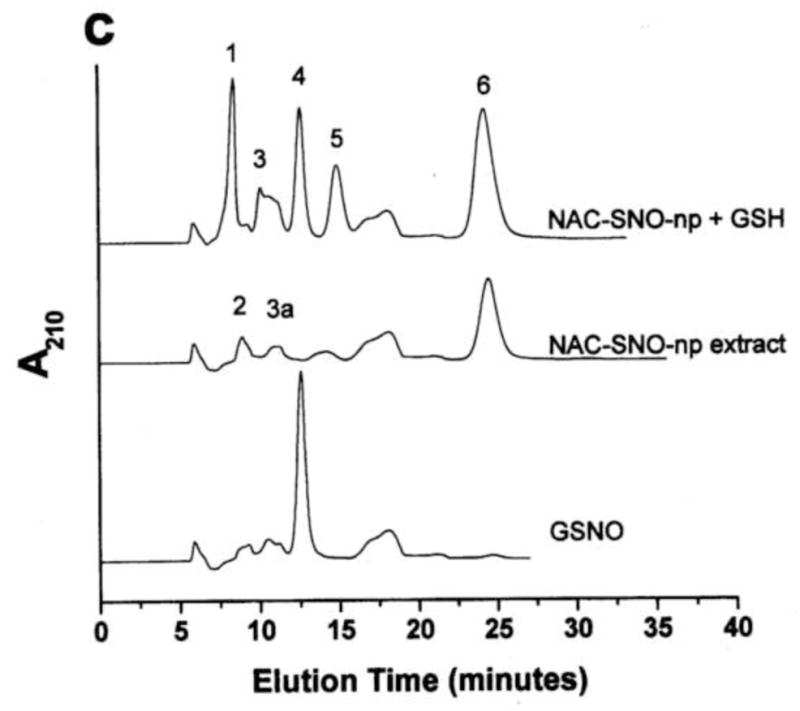
NO-np (A), SNO-np (B), and NAC-SNO-np-2 (C) were incubated at 20 mg/ml in 0.5 mM DTPA/PBS, pH 7.4 for 1 hour at room temperature shielded from light in the absence and presence of GSH (20 mM) while mixing on a lab rotator. Supernatants were diluted (100x for extracts and 50x for GSH-reaction mixtures) and analyzed by RPHPLC as described in the text. GSH-reaction mixtures of all the particles displayed a peak corresponding to GSNO, which was absent in the respective extracts (in the absence of GSH). Peaks identities are as follows: 1 = GSH, 2 = nitrite, 3 = GSSG, 3a = nitrate, 4 = GSNO, 5 = non-characterized oxidized product of NAC-SNO, 6 = NAC-SNO.
