Abstract
Maesabalide III (MB-III), an oleane triterpene saponin isolated from the Vietnamese plant Maesa balansae, is a new antileishmanial lead compound whose activity against Leishmania donovani (MHOM/ET/67/L82) in groups of five golden hamsters was evaluated after administration of a single subcutaneous dose on either day 1 (prophylactic treatment) or day 28 (curative treatment) after infection. Liposomal amphotericin B (AmBisome), administered intravenously at 5 mg/kg of body weight, was used as the reference drug. Amastigote burdens in liver, spleen, and bone marrow were determined either 7 days (early effects) or 56 days (late effects) after treatment. Prophylactic administration of MB-III at 0.2 mg/kg reduced liver amastigote burdens by 99.8 and 83% within 7 and 56 days after treatment, respectively. In the latter group, however, all animals became ill and some died. Both MB-III at 0.8 mg/kg and liposomal amphotericin B were 100% effective against liver stages, but clearance from the spleen and bone marrow was not achieved. Curative administration of MB-III at 0.2 and 0.4 mg/kg was not protective, as no survivors were left at the termination of the experiment on day 84. Despite the high level of reduction of the liver amastigote burden after treatment with MB-III at 0.8 mg/kg (94.2%) or liposomal amphotericin B (99.4%), clinical protection could not be obtained in either group, with two deaths occurring and the residual liver burdens persisting. It is concluded that administration of a single dose of MB-III at 0.8 mg/kg has efficacy potential comparable to that of a single dose of liposomal amphotericin B at 5 mg/kg and is therefore considered a promising new antileishmanial lead compound. However, multiple-dose pharmacological, toxicological, and pharmacokinetic studies are still needed before it can become a valid drug candidate for development.
Among other tropical diseases, insufficient resources are being allocated to tackle leishmaniasis (15). The emergence of resistance to the first-line drugs (19) and the fact that the drugs available at present do not adequately cover the prime requirements for effective disease control (4) explain why new drug discovery must remain a pivotal objective (9). We recently demonstrated that olenane triterpene saponins (PX-6518) are promising new antileishmanial lead structures (10). By using the Leishmania donovani-infected BALB/c mouse model, administration of a single subcutaneous dose at 0.4 mg/kg of body weight day 1 after infection reduced liver amastigote burdens by about 95% within 7 days of treatment. Although the BALB/c model is highly validated for drug screening purposes, it does not reproduce the progressive disease features that are seen in human visceral leishmaniasis (VL) (14). The golden hamster is regarded to be more sensitive to progressive VL and is a better model for prediction of the effects of drugs on progressive VL (11, 16) and was therefore selected as the experimental host in the present study. From advanced characterization of the individual constituents of PX-6518, maesabalide III (MB-III) (Fig. 1) was retained as a potential drug development candidate (10). Although multiple-dose treatment schedules are adopted in standard clinical practice, single-dose regimens for MB-III and liposomal amphotericin B (AmBisome) were envisaged in the present study because a high degree of efficacy is already obtained after administration of a single dose (10) and because this provided a more accurate basis for comparative assessment of their efficacies. Among the commercial liposomal formulations of amphotericin B, AmBisome was shown to have the best antileishmanial potential under experimental conditions (20) and in clinical practice as a low, single-dose treatment (18).
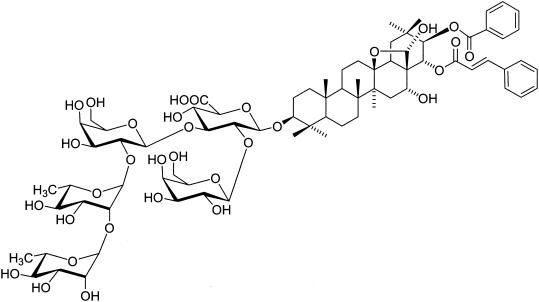
FIG. 1.
Structure of the triterpene oleane saponin MB-III isolated from the leaves of the Vietnamese plant M. balansae.
MATERIALS AND METHODS
Parasite.
Amastigotes of a drug-sensitive L. donovani strain (MHOM/ET/67/L82) were harvested from the spleens of 6-week-infected donor hamsters (Mesocrisetus auratus). The inoculum for infection was prepared as described previously (10) and involved purification of amastigotes from homogenized spleen by a low-speed centrifugation cycle at 25 × g (10 min at 4°C) to remove tissue particles, followed by a high-speed centrifugation cycle at 1,000 × g. The number of amastigotes recovered was determined as described by Stauber (17).
Compounds.
The methods for isolation of the active constituents from the leaves of M. balansae (PX-6518) and purification to the individual maesabalide saponins were described previously (8, 10). PX-6518 was used in the pilot toxicity experiment (experiment 1) because the supplies of MB-III were not sufficient. PX-6518 and MB-III were formulated in isotonic saline at 10 mg/ml and were sterilized through a 0.22-μm-pore-size filter (Millipore). Formulations for injection were prepared by further dilution in saline. Liposomal amphotericin B (AmBisome; Nextar) was diluted in its appropriate vehicle to prepare a 2.5-mg/ml stock solution.
Experimental protocol. (i) Experiment 1: toxicity and efficacy titration of PX-6518 in hamsters infected with L. donovani.
Thirty hamsters (males and females; weight, 80 to 100 g) were intracardially infected with 107 amastigotes on day 0 of the experiment. Treatment with PX-6518 was started on day 1 or day 40 after infection and consisted of a single subcutaneous (s.c.) dose (dose range for efficacy evaluation, 0.63 to 2.5 mg/kg) or a 5-day intraperitoneal (i.p.) dosing regimen (dose range for toxicity evaluation, 2.5 to 40 mg/kg). During and after treatment, the animals were monitored for the appearance of adverse clinical signs, and the efficacies of the treatments were evaluated by determining the amastigote burdens in the livers at 7 days posttreatment (Table 1).
TABLE 1.
Toxicity and efficacy titration of PX-6518 in the L. donovani hamster model
| Treatment group
|
Effects of treatment with PX-6518
|
||||
|---|---|---|---|---|---|
| Regimen | Dose (mg/kg) | Time of treatment start (dpia) | Animal no. | % Reductionb | Clinical observations |
| Daily i.p. dose for 5 consecutive days | 40 | 1 | 3 | 100 | No signs of drug intolerance |
| 20 | 1 | 3 | 100 | ||
| 10 | 1 | 3 | 100 | ||
| 5 | 40 | 1 | NDc | Complete clinical recovery within 2 mo after treatment; untreated control died at 65 dpi due to clinical disease | |
| 2.5 | 40 | 1 | ND | ||
| 0 | 40 | 1 | ND | ||
| Single s.c. dose | 2.5 | 1 | 6 | 100 | No signs of drug intolerance |
| 1.25 | 1 | 6 | 100 | ||
| 0.63 | 1 | 6 | 99.9 | ||
dpi: day(s) postinfection.
Amastigote burdens in the liver at 7 days posttreatment (LDU in untreated controls, 4,515 ± 1,418).
ND, not done.
(ii) Experiment 2: lowest active dose of MB-III compared to that of liposomal amphotericin B.
One hundred hamsters (males and females; weight, 150 to 200 g) were randomly allocated into 20 groups of five animals each and were infected intracardially with 107 amastigotes on day 0 of the experiment. Treatment with MB-III or liposomal amphotericin B was performed at day 1 postinfection (prophylactic treatment) or was delayed until day 28 postinfection (curative treatment). The efficacy of the treatment was evaluated by monitoring the parasite burdens in the target organs (liver, spleen, and bone marrow) at either day 7 (early drug effects [see Table 2 ]) or day 56 (late drug effects [see Table 3]) after treatment.
TABLE 2.
Early effectsa of treatment with MB-III and liposomal amphotericin B on acute and established L. donovani infections in hamsters
| Treatment groupb and treatment | Body wt (g) on day 0c | % Body wt gain at day 7c | Length of survival (day postinfection) | Liver wt and burdens
|
Other target organs
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Wt (mg)a | LDU | % Reduc- tion | Spleen wt (mg)c | No. of animals with positive:
|
|||||
| Spleen | Bone marrow | ||||||||
| Treatment of acute infection on day 1 postinfection | |||||||||
| Control (no treatment) | 191 ± 32 | 5 ± 4 | 8 | 10,722 ± 2,051 | 11,237 ± 2,422 | 411 ± 115 | 5 | 5 | |
| MB-III at 0.2 mg/kg s.c. | 161 ± 33 | 5 ± 4 | 8 | 8,015 ± 2,409 | 22 ± 40 | 99.8 | 502 ± 111 | 5 | 4 |
| MB-III at 0.4 mg/kg s.c. | 169 ± 22 | 5 ± 2 | 8 | 8,238 ± 1,311 | 1 ± 3 | 100 | 417 ± 195 | 5 | 0 |
| MB-III at 0.8 mg/kg s.c. | 197 ± 12 | 1 ± 2 | 8 | 8,138 ± 1,311 | 0 ± 0 | 100 | 351 ± 166 | 0 | 0 |
| Amphotericin Bd at 5 mg/kg i.v.e | 176 ± 43 | 3 ± 7 | 8 | 8,879 ± 1,896 | 0 ± 0 | 100 | 326 ± 145 | 0 | 1 |
| Treatment of chronic infection on day 28 postinfection | |||||||||
| Control (no treatment) | 162 ± 35 | −24 ± 14 | 35 | 11,026 ± 2,670 | 51,489 ± 37,220 | 889 ± 200 | 5 | 5 | |
| MB-III at 0.2 mg/kg s.c. | 187 ± 30 | −33 ± 19 | 35 | 11,412 ± 751 | 28,130 ± 6,476 | 45.4 | 1,195 ± 327 | 5 | 5 |
| MB-III at 0.4 mg/kg s.c. | 180 ± 23 | −29 ± 14 | 35 | 11,284 ± 770 | 16,176 ± 11,167 | 68.6 | 1,030 ± 345 | 5 | 5 |
| MB-III at 0.8 mg/kg s.c. | 158 ± 29 | −34 ± 16 | 35 | 8,752 ± 1,769 | 3,008 ± 2,248 | 94.2 | 692 ± 206 | 2 | 5 |
| Amphotericin B at 5 mg/kg i.v. | 163 ± 42 | −22 ± 8 | 35 | 9,471 ± 2,136 | 331 ± 442 | 99.4 | 681 ± 290 | 5 | 5 |
Early effects of treatment were determined by necropsy at day 7 posttreatment.
Groups of five animals each (males and females) received a single dose.
Values are means ± standard deviations.
Liposomal amphotericin B (AmBisome).
i.v., intravenously.
TABLE 3.
Late effectsa of treatment with MB-III and liposomal amphotericin B on acute and established L. donovani infections in hamsters
| Treatment groupb | Body wt (g) on day 0c | % Body wt gain at day 56c | Survival
|
Liver wt and burdensd
|
Other target organsd
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of survivors at necropsy | Day post- infectionc | Wt (mg)c | LDUc | % Reduc- tion | Spleen wt (mg)c | No. of animals with positive:
|
||||
| Spleen | Bone marrow | |||||||||
| Treatment of acute infection on day 1 postinfection | ||||||||||
| Control (no treatment) | 188 ± 38 | −69 ± 6 | 2 | 53 ± 5 | 8,056 ± 1,382 | 100,147 ± 58,715 | 828 ± 66 | 2 | 2 | |
| MB-III at 0.2 mg/kg s.c. | 161 ± 16 | −52 ± 17 | 2 | 54 ± 3 | 7,082 ± 2,642 | 17,012 ± 21,508 | 83.0 | 1,477 ± 1,160 | 2 | 2 |
| MB-III at 0.4 mg/kg s.c. | 200 ± 12 | −8 ± 7 | 3 | 51 ± 7 | 10,614 ± 2,663 | 172 ± 297 | 99.8 | 2,007 ± 1,563 | 3 | 3 |
| MB-III at 0.8 mg/kg s.c. | 176 ± 30 | 6 ± 9 | 4 | 54 ± 5 | 9,608 ± 2543 | 0 ± 0 | 100 | 666 ± 195 | 4 | 0 |
| Amphotericin Be at 5 mg/kg i.v.f | 163 ± 29 | 17 ± 8 | 5 | 56 ± 0 | 9,317 ± 1302 | 0 ± 0 | 100 | 629 ± 456 | 4 | 2 |
| Treatment of chronic infection on day 28 postinfection | ||||||||||
| Control (no treatment) | 142 ± 18 | NAg | 0 | 50 ± 7 | NDh | ND | ND | ND | ND | |
| MB-III at 0.2 mg/kg s.c. | 157 ± 30 | NA | 0 | 48 ± 12 | ND | ND | ND | ND | ND | ND |
| MB-III at 0.4 mg/kg s.c. | 146 ± 23 | NA | 0 | 68 ± 14 | ND | ND | ND | ND | ND | ND |
| MB-III at 0.8 mg/kg s.c. | 158 ± 39 | −36 ± 13 | 3 | 78 ± 9 | 10,067 ± 1,972 | 5 ± 6 | ND | 475 ± 181 | 0 | 0 |
| Amphotericin B at 5 mg/kg i.v.f | 173 ± 35 | −38 ± 26 | 3 | 81 ± 4 | 34,901 ± 39,093 | 1,347 ± 2,298 | ND | 572 ± 224 | 0 | 0 |
Early effects of treatment were determined by necropsy at day 56 posttreatment.
Groups of five animals each (males and females) received a single dose.
Values are means ± standard deviations.
Only survivers were evaluated.
Liposomal amphotericin B (AmBisome).
i.v., intravenously.
NA, not applicable.
ND, not done.
End points for efficacy evaluation. (i) Parasite burdens.
The livers of individual animals were weighed, and impression smears were prepared for Giemsa staining and microscopic enumeration of the number of amastigotes per liver cell. The total amastigote burdens were calculated as Leishman Donovan units (LDU; LDU is the mean number of amastigotes per liver cell times the liver weight [in milligrams]) (3), and an LDU reduction of at least 80% was adopted as the minimal criterion for drug efficacy. Because of a very low level of infection in the spleen and the bone marrow after treatment, viable amastigotes in these organs were traced qualitatively by the promastigote transformation test, as described previously (10). Collection of target organs and determination of amastigote burdens were not done for animals that succumbed due to severe clinical disease.
(ii) Body weight.
The animals in experiment 2 were weighed at the time of the treatment (day 1) and again at the end of the experiment (on day 7 or day 56 posttreatment) to monitor the effects of the medication and the infection on their general health status.
RESULTS
Experiment 1: toxicity and efficacy titration of PX-6518.
In the 5-day repeated-dosing scheme, i.p. dosing was used to allow administration of larger inoculum volumes together with a better overall systemic absorption potential. No clinical signs of drug intolerance were observed during or after treatment with 40 mg/kg for 5 days, and no amastigotes could be recovered from the liver (Table 1). The three animals infected for 40 days showed clinical signs of disease (rough hair coat, dull appearance, body weight loss) at the time of treatment. While the untreated control animal succumbed 25 days later, both animals treated with MB-III (2.5 and 5 mg/kg, respectively) fully recovered within 2 months. The single-dose efficacy titration revealed that liver infection was adequately controlled down to 0.63 mg/kg (99.9% reduction in amastigote burdens compared to those for the untreated controls), which formed the basis for selection of the MB-III dose in experiment 2, i.e., between 0.2 and 0.8 mg/kg.
Experiment 2: lowest active dose of MB-III compared to that of liposomal amphotericin B.
While the treatment in experiment 1 was given as a prophylactic dose within 24 h of the artificial infection, the experiment 2 also included treatment of well-established infections by the postponement of treatment until 4 weeks after infection. A second variable was the discrimination between the early effects (7 days; Table 2) and the late effects (56 days; Table 3) of drug action.
The early and late effects after prophylactic treatment on day 1 postinfection were fairly comparable: a single 0.2-mg/kg dose of MB-III resulted in 99.8 and 83% reductions in liver amastigote burdens at 7 and 56 days after treatment, respectively. The severity of infection in the untreated controls increased dramatically between day 7 and day 56, as illustrated by three deaths, relative weight loss of about 69% in the survivors, and a 10-fold increase in the liver amastigote burdens (from 10,722 ± 2,051 LDU at day 7 to 100,147 ± 58,715 LDU at day 56). Despite the apparently adequate parasitological control by MB-III at 0.2 mg/kg (83% reduction in liver amastigote burdens), deaths did occur and the survivors showed considerable weight loss (52%). Treatment with MB-III at 0.8 mg/kg resulted in good parasitological and clinical control, with a 100% reduction in liver amastigote burdens. However, one animal died on day 46 and the overall body weight gain was low (6 ± 9 g). Adequate control was obtained with liposomal amphotericin B (100% reduction of liver amastigote burdens, 100% survival, and no weight loss); but as was also the case for MB-III, infection of the spleen and, to a lesser extent, the bone marrow as well was not prevented. No major effects on liver and spleen weights were noted.
The infection became much more difficult to control if the treatment was delayed until 28 days postinfection (curative treatment). By day 35, control animals already showed severe liver infection (51,489 ± 37,220 LDU), poor health status, and a body weight loss of about 24%. Control of liver infection was obtained with MB-III at 0.8 mg/kg and liposomal amphotericin B, with 94.2 and 99.4% reductions within 7 days of treatment, respectively. However, a net body weight loss was noted in all animals, and both spleen and bone marrow remained positive for amastigotes. MB-III at 0.2 and 0.4 mg/kg was not adequately effective (reductions of amastigote burdens, <80%); and when the infection was allowed to proceed until day 84 after infection (56 days posttreatment), a large number of animals succumbed to the infection and did not complete the experiment. No survivors were left among the untreated controls or the groups treated with MB-III at 0.2 and 0.4 mg/kg. Only three of the five animals in each of the groups receiving MB-III at 0.8 mg/kg and liposomal amphotericin B completed the experiment. Among the survivors, the levels of liver infection had become very low and no residual infection could be detected in the spleen or bone marrow. At the termination of the experiment, the animals still showed a net body weight loss but were clearly recovering.
DISCUSSION
Our recent discovery of the new antileishmanial lead compound PX-6518, a mixture of triterpene oleane saponins isolated from the plant M. balansae (8, 10), urged more in-depth assessments of whether this lead compound could become a valid drug candidate. The fact that PX-6518 is extracted from plant material as a mixture and cannot be synthesized by chemical means becomes a particular hurdle with regard to the regulatory requirements for drug development, in which batch-to-batch consistency and the need for a fully characterized marker compound remain pivotal requirements (5, 6). To address both of these requirements, MB-III was retained as single lead candidate because it has the most potent in vitro and in vivo activities and among the components of the PX-6518 mixture is the largest contributor to pharmacological activity (10). The toxicity and dose titration studies with PX-6518 and pure MB-III were performed with the golden hamster, as this animal is believed to be more appropriate than the BALB/c mouse for evaluation of the long-term effects of drugs against chronic VL (11). Liposomal amphotericin B, at present the best available drug with clinically confirmed efficacy in humans when given as a low, single-dose regimen (18), became the obvious target for the comparative efficacy evaluation.
The toxicity-titration study (Table 1) showed that PX-6518 is well tolerated after the administration of five i.p. doses of 40 mg/kg each. It must be noted, however, that only clinical parameters were monitored. In addition, full recovery from clinical disease was noted after i.p. administration with five doses at 2.5 mg/kg each. The single-dose efficacy of PX-6518 at 0.63 mg/kg (Table 1) or MB-III at 0.2 mg/kg (Table 2) was fully comparable to that of the lowest active dose of 0.4 mg/kg in the BALB/c model (10), thereby establishing the comparability of both models of acute VL. However, adequate control of established infections was more problematic: MB-III at 0.8 mg/kg and liposomal amphotericin B were capable of controlling liver infection (>90% reduction), but weight losses and deaths ultimately could not be prevented (Table 3). These observations are in line with those of other studies evaluating the efficacy of liposomal amphotericin B against experimental VL infections. However, they nearly all relate to the BALB/c mouse model of acute infection, and few adopted a single-dose regimen (20) or discriminated between early and late drug effects (7, 12, 13). A consistent observation is the difficulty of treatment of chronic infection and the inability to clear the infection from the spleen and bone marrow, even after the administration of multiple high doses, thereby leaving a residual parasite population that may form the basis for relapses after elimination of the drug.
A cell-targeting phenomenon forms the basis for the enhanced antileishmanial activity observed with liposomal amphotericin B compared to that observed with free drug (1, 2). At this stage, there is as yet no evidence to suggest that MB-III would also have a similar cell-targeting potential, but its selective toxicity toward reticuloendothelial cells at least suggests this possibility (10).
In summary, this study shows that MB-III has potential as a potent therapy for VL and that its potency is comparable to that of liposomal amphotericin B. In view of the present need for new antileishmanial drugs, additional studies are planned to assess whether MB-III could become a valid candidate for drug development. The study package will include evaluations of the efficacies of multiple-dose treatments against visceral and (muco)cutaneous Leishmania species and drug-resistant field isolates, mode-of-action studies, and toxicology and pharmacokinetic studies with laboratory animals and humans.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and the Belgian Directorate for Overseas Development (DGOS). Cos Paul is a postdoctoral researcher of the Fund for Scientific Research (FWO; Flanders, Belgium).
REFERENCES
- 1.Agrawal, A. K., A. Agrawal, A. Pal, P. Y. Guru, and C. M. Gupta. 2002. Superior chemotherapeutic efficacy of amphotericin B in tuftsin-bearing liposomes against Leishmania donovani infection in hamsters. J. Drug Target. 10:41-45. [DOI] [PubMed] [Google Scholar]
- 2.Berman, J. D., W. L. Hanson, W. L. Chapman, C. R. Alving, and G. Lopez-Berestein. 1986. Antileishmanial activity of liposome-encapsulated amphotericin B in hamsters and monkeys. Antimicrob. Agents Chemother. 30:847-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, D. J. 1977. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin. Exp. Immunol. 30:130-140. [PMC free article] [PubMed] [Google Scholar]
- 4.Bryceson, A. 2001. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop. Med. Int. Health 6:928-934. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, Y. Y. 2000. Guidance for industry. Botanical drug products, draft guidance. Center for Drug Evaluation and Research, Food and Drug Administration, Rockville, Md.
- 6.Committee for Proprietary Medicinal Products-Committee for Veterinary Medicinal Products. 2001. Note for guidance on specifications: test procedures and acceptance criteria for herbal drugs, herbal drug preparations and herbal medicinal products. EMEA/CVMP/815/00. Committee for Proprietary Medicinal Products-Committee for Veterinary Medicinal Products, London, United Kingdom.
- 7.Gangneux, J. P., A. Sulahian, Y. J. Garin, R. Farinotti, and F. Derouin. 1996. Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisome. Antimicrob. Agents Chemother. 40:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germonprez, N., L. Van Puyvelde, L. Maes, M. Van Tri, and N. De Kimpe. 2003. New pentacyclic triterpene saponins with strong anti-leishmanial activity from the leaves of Maesa balansae. Tetrahedron 60:219-228. [Google Scholar]
- 9.Guerin, P., J. P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 10.Maes, L., D. Vanden Berghe, N. Germonprez, L. Quirijnen, P. Cos, N. De Kimpe, and L. Van Puyvelde. 2003. In vitro and in vivo activity of a triterpenoid saponin extract (PX-6518) from the plant Maesa balansae against visceral leishmania species. Antimicrob. Agents Chemother. 48:130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melby, P. C., B. Chandrasekar, W. Zhao, and J. E. Coe. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 166:1912-1920. [DOI] [PubMed] [Google Scholar]
- 12.Mullen, A. B., A. J. Baillie, and K. C. Carter. 1998. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of a nonionic surfactant formulation of sodium stibogluconate with those of three proprietary formulations of amphotericin B. Antimicrob. Agents Chemother. 42:2722-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullen, A. B., K. C. Carter, and A. J. Baillie. 1997. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob. Agents Chemother. 41:2089-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, H. W., H. Masur, and J. S. Keithly. 1982. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J. Immunol. 129:344-350. [PubMed] [Google Scholar]
- 15.Pecoul, B., P. Chirac, P. Trouiller, and J. Pinel. 1999. Access to essential drugs in poor countries: a lost battle? JAMA 281:361-367. [DOI] [PubMed] [Google Scholar]
- 16.Requena, J. M., M. Soto, M. D. Doria, and C. Alonso. 2000. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet. Immunol. Immunopathol. 76:269-281. [DOI] [PubMed] [Google Scholar]
- 17.Stauber, L. A. 1966. Characterisation of strains of Leishmania donovani. Exp. Parasitol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 18.Sundar, S., and M. Rai. 2002. Advances in the treatment of leishmaniasis. Curr. Opin. Infect. Dis. 15:593-598. [DOI] [PubMed] [Google Scholar]
- 19.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 20.Yardley, V., and S. L. Croft. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13:243-248. [DOI] [PubMed] [Google Scholar]