Abstract
Anopheles sinensis is an important vector of Plasmodium vivax in Southeast Asia. To facilitate population genetic and genomic studies of An. sinensis, we developed a standard cytogenetic photomap for this species. The polytene chromosomes were straightened and divided into 39 numbered divisions and 116 lettered subdivisions. The chromosomal localizations of 13 DNA probes were determined by fluorescent in situ hybridization. The comparison of the physical map of An. sinensis with the genome map of An. gambiae revealed a whole-arm autosomal translocation between the two species. Specifically, the 2R arm of An. gambiae corresponds to the 3R arm of An. sinensis, while the correspondences of the other chromosome arms are preserved. We mapped the breakpoints of the polymorphic paracentric chromosomal inversion 3Ra to subdivisions 28A and 31A. The standard cytogenetic map developed in this study will be useful for detailed comparative genome mapping and population genetic studies of An. sinensis.
Introduction
Anopheles sinensis Wiedemann 1828 is a member of the Hyrcanus group that belongs to the Myzorhynchus series within the subgenus Anopheles (Harbach, 2004). It has a wide geographical distribution in Southeast Asia (Hay et al., 2010). Within the distribution range of An. sinensis, its importance as a vector of Plasmodium vivax and filariasis has been well recognized (Sinka, 2011). In Korea, An. sinensis is considered a major vector species of vivax malaria and is responsible for the recurrence of malaria in recent years (Foley et al., 2009; Rueda et al., 2010). In central China, it is a principal vector that plays an important role in the epidemiology of malaria in poor endemic areas as well as in occasional epidemics in certain localities (Ma et al., 2011). Due to its wide distribution, An. sinensis has been an important historical vector of malaria in Japan and Taiwan (Rueda et al., 2005). Additionally, Japanese Encephalitis Virus has been isolated from this species, indicating some degree of its susceptibility to the virus (Liu et al., 2013).
Because of its importance as a vector, An. sinensis is of great interest for genomic and cytogenetic studies. This species has a 2n=6 chromosome complement typical to Anopheles. Sex chromosomes, X or X and Y in males, and two autosomes (2 and 3), comprise the chromosomal complement of this species. As the Y chromosome does not polytenize in An. sinensis, only five well-defined polytene chromosome arms can be visualized in its salivary glands. The sex chromosome (X) as well as the right and left arms of chromosomes 2 (2R and 2L) and 3 (3R and 3L) are easily recognized in a typical polytene preparation. To date, several chromosome maps have been developed for An. sinensis. The first description of banding patterns of polytene chromosomes was done using a Japanese strain, however, this map was not published (Kanda, 1968). The first drawn map of An. sinensis chromosomes from salivary glands was developed for an Indian strain, but the map was without divisions and subdivisions (Sharma, 1971). Later work on a Chinese strain of An. sinensis yielded a drawn map and photomap with 39 numbered divisions and 116 lettered subdivisions (Ye, et al., 1981). Additionally, several cytogenetic maps have been constructed for different wild Chinese strains; these maps have been extensively used to identify various geographic populations of An. sinensis in China (Xu, et al., 1981; Li, et al., 1983; Ye, et al., 1983; Ye, et al., 1985). Cytogenetic map of An. sinensis have been useful for distinguishing sibling species within the Hyrcanus group. For example, An. hyrcanus and An. anthropophagus can be differentiated from An. sinensis by one fixed inversion on the 2L arm (Xu, et al., 1990; Xu, et al., 1991). However, all available chromosome maps for An. sinensis are either drawn or made from chromosomal images that were not straightened.
A cytogenetic map made from high-resolution chromosomal images is a necessary step toward physical mapping of the malaria mosquito genomes and comparative genome analyses. The availability of physical maps for An. gambiae and An. stephensi has provided valuable information for improving the whole genome assembly and understanding genome organization and evolution (Holt et al., 2002; Sharakhova et al., 2010; Xia et al., 2010; Sharakhova et al., 2011). Comparative physical mappings have revealed preserved synteny at the chromosomal arm level in Anopheles mosquitoes, suggesting the frequent occurrence of whole-arm translocations in evolution (Sharakhov, et al., 2008). Multiple whole-arm translocations occurred within the subgenera Cellia and between Cellia and Nyssorhynchus (Cornel and Collins, 2000; Sharakhov et al., 2002; Sharakhova et al., 2006). Recent studies have demonstrated that the sex (X) chromosome evolves faster than autosomes, and the 2R arm has a higher rate of inversion fixations than other autosomal arms (Xia et al., 2010; Sharakhova et al., 2011). The lack of a physical map for An. sinensis has excluded it from comparative genomics studies with other mosquitoes. Moreover, to better understand genetic determinants of vectorial capacity, the National Institutes of Health (NIH) has funded the sequencing of the genomes and transcriptomes of 16 Anopheles species (http://www.broadinstitute.org/annotation/genome/anopheles). Anopheles sinensis is among the 16 species whose genome will be sequenced (https://agcc.vectorbase.org/index.php/Anopheles_Genome_Cluster). The development of genome-mapping tools for An. sinensis presents an opportunity to unite cytogenetic, sequencing and transcriptome data to greatly advance knowledge of this important disease vector.
In this study, we used high-resolution chromosomal images to develop a standard photomap of polytene chromosomes from the salivary gland for An. sinensis. Inversion breakpoints of a paracentric inversion from the Shanghai laboratory strain were mapped onto the 3R arm. We also hybridized 13 DNA probes conserved with An. gambiae to chromosomes of An. sinensis. Gene order comparisons between An. gambiae and An. sinensis strongly suggest a whole-arm translocation between the autosomes of these species.
Materials and methods
Mosquito strain and chromosome preparation
The Shanghai laboratory strain of An. sinensis (National Institute for Parasitic Diseases, Chinese Center for Disease Control and Prevention, Shanghai, China) was used in this study. Large, early 4th-instar larva were selected and fixed in Carnoy’s solution (Methanol: Glacial Acetic Acid = 3:1) at −20°C.
To prepare the polytene chromosome preparations, the larvae were removed from the vials and placed into a drop of Carnoy’s solution on a microscope slide under an Olympus stereomicroscope (Olympus Corporation, Shinjuku-ku Tokyo, Japan). After the head was gently removed from the thorax, a needle was inserted at the middle rear of the thorax directly underneath the cuticle, and gently moved forward to break the cuticle along the mid-dorsal line. Next, the thorax was carefully opened up, and the salivary glands were separated from connecting tissue. One drop of 50% propionic acid was then added to the clean glands, and they were immediately covered with a glass cover slip. The chromosomes were flattened by gently tapping with a pencil eraser tip against the cover slip. An Olympus BX43 phase-contrast microscope (×1000) (Olympus Corporation, Shinjuku-ku Tokyo, Japan) was used to observe the banding patterns of the polytene chromosomes. Slides with good chromosomes were dipped into liquid nitrogen, their cover slips were removed, and they were dehydrated in 50%, 70%, 90%, and 100% ethanol. Chromosomes with clear banding patterns were imaged with an Olympus BX43 microscope with a DP72 digital camera and cellSens imaging software (Olympus Corporation, Shinjuku-ku Tokyo, Japan). The best 100 chromosome images from a total of approximately 500 were used to construct the cytogenetic map. Chromosome images were processed using Adobe Photoshop. Homozygous and heterozygous inversions of 3Ra were scored according to the chromosomal map presented in this article.
Fluorescence in situ hybridization
Primers were designed with the Primer3 Program (Rozen and Skaletsky, 2000) based on sequences from the An. gambiae genome (www.vectorbase.org) and our unpublished sequence data. The genomic DNA of An. sinensis was extracted from live 4th-instar larva with a DNeasy Blood & Tissue Kit (QIAGEN GmbH, Germany). This was used as a template for PCR. The PCR products were gel purified with a QIAquick Gel Extraction Kit (QIAGEN GmbH, Germany). DNA was labeled either with Cy3.5-AP3-dUTP or with Cy5.5-AP3-dUTP (GE Healthcare UK Ltd., Buckinghamshire, England) using a Random Primed DNA Labeling Kit (Roche Applied Science). The in situ hybridization procedure was conducted as previously described (Sharakhova et al., 2006). The chromosomes were washed in 0.2X SSC (Saline-Sodium Citrate: 0.03 M Sodium Chloride, 0.003 M Sodium Citrate), counterstained with YOYO-1, and mounted in DABCO. Fluorescent signals were detected and recorded with a Zeiss LSM 710 Laser Scanning Microscope (Carl Zeiss Microimaging GmbH, Germany).
Results
Polytene chromosome photomap for An. sinensis
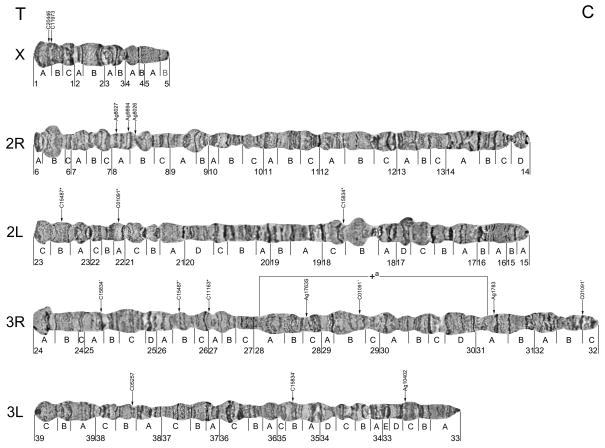
The polytene chromosome complement in the salivary glands of An. sinensis comprises one short sex (X) chromosome and four autosomal arms. We analyzed chromosome banding patterns and developed a photomap of straightened An. sinensis polytene chromosomes with 39 numbered divisions and 116 lettered subdivisions (Fig. 1, Fig. S1). In general, we adopted the nomenclature and boundaries used for divisions and subdivisions in two previously published drawn maps (Li, et al., 1983; Ye, et al., 1983). Some boundaries were slightly modified to make the map more suitable for genome mapping. For example, divisions and subdivisions started with either light band or dark band, and divisions were divided evenly for making subdivisions. The landmarks that are useful for arm recognition are described below.
Fig. 1. A cytogenetic map for An. sinensis showing the location of one polymorphic inversion on the 3R chromosomal arm and physically mapped DNA markers.
*indicates major signal, ’ indicates multiple signal. T indicates telomere region, C indicates centromere region.
As is typical for the genus Anopheles, the X chromosome is the shortest arm of the polytene complement (Fig. 1, Fig. S1). The telomeric region 1A can be easily recognized by a dark, granulated telomeric end. The centromeric end of the X chromosome has a dark narrow band in the most proximal part of region 5B. A bulbous region in 1C-2A with a dark band in the middle of the bulb can be considered an additional landmark for the X chromosome.
Autosome 2 is metacentric, meaning that it has two arms of almost equal size (Fig. 1, Fig. S1). The telomeric end of 2R has two distinct narrow bands at the tip and a large round puff in subdivision 6B. An additional puff in region 9A–B, a dark wide band in region 9A, and three narrow bands in region 9B can be used as supplemental landmarks for the 2R arm. In addition, regions 11B and 11C, identified by the existence of two series of closely located thin dark bands, provide landmarks for the middle part of the arm. The centromeric end has a small puff in region 14D. The 2L arm usually has a dark, granulated, flared telomeric end. The existence of a large light puff in region 18B, a dark puff in region 17D, and a diffuse area with three dark bands in (18A) provides supporting identifying characteristics for the confirmation of a 2L arm. The pericentromeric area is easily recognized by an underpolytenized (low level of polyteny) dark band in region 15A and three dark bands in 15B.
The 3R arm is the longest of four autosomal arms (Fig. 1, Fig. S1). The telomeric area of 3R is light in color and flared. In 26C-27A, the presence of two large puffs with a pair of large bands in the middle is a consistent feature of 3R. The existence of two large dark bands in regions 31A and 31B can be considered additional landmarks of this arm. Close to the centromere in region 32A of the 3R arm, there are a series of dark bands.
The relative length of the 3L arm is considerably shorter than the rest of the autosomal arms, and the telomeric end can be easily distinguished by a puff with one dark underpolytenized dot at the tip (Fig. 1, Fig. S1). Several large puffs are located along the chromosome. The most prominent banding patterns are in the middle part of the arm, which are well marked with two closely located dark bands and two large puffs in region 36C-37B. The pericentromeric area has three dark bands in region 33B.
Chromosome arm homology between An. sinensis and An. gambiae
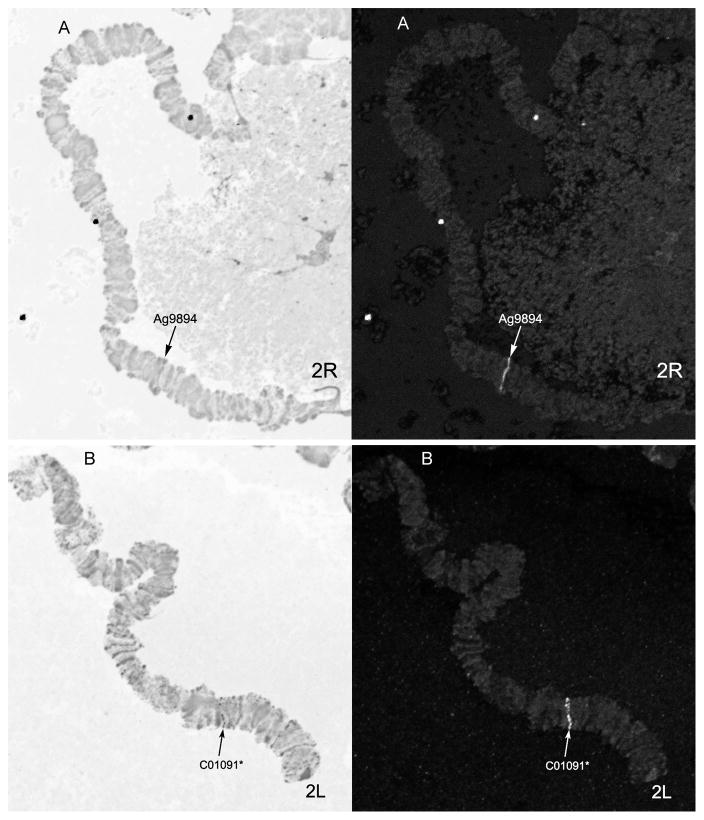
A total of 13 DNA probes from PCR-amplified fragments of An. sinensis genomic DNA has been used for in situ hybridization. Primers for six probes were designed based on the An. gambiae DNA sequences available from VectorBase (www.vectorbase.org). The other seven probes were inferred from our unpublished genome sequence data of An. sinensis. Purified PCR products were labeled with dUTP-Cy3.5 and dUTP-Cy5.5 and then hybridized to the polytene chromosomes of An. sinensis. The exact location of each signal on the chromosome was mapped to the An. sinensis cytogenetic map (Fig. 1 and Fig. 2). A major signal was defined as occurring when a clone had multiple hybridization signals but only one fluorescent signal that was much stronger than the rest. The locations of the sequences in the An. gambiae genome were determined by BLASTN (https://www.vectorbase.org/blast) (Table 1).
Fig. 2. Fluorescent in situ hybridization of two DNA probes labeled with Cy3.5 and Cy5.5 on 2R and 2L chromosomal arms: Ag9894(A) and C01091 (B).
Right panels show the banding pattern of the chromosomes. Left panel show fluorescence due to hybridization. Arrow indicates signals of hybridization; * indicates major signal.
Table 1.
DNA probes used for in situ hybridization with the An. sinensis polytene chromosomes
| Clone name | Accession no. | Location in An. sinensis | Location in An. gambiae | |
|---|---|---|---|---|
| 1 | C20446 | unpublished | X:1A | X:4C(6e-107) |
| 2 | C11973 | unpublished | X:1A | X:4C(2e-120) |
| 3 | Ag8027 | AGAP008027 | 2R:8A | 3R:29D |
| 4 | Ag9894 | AGAP009894 | 2R:8A | 3R:35D |
| 5 | Ag8026 | AGAP008026 | 2R:8B | 3R:29D |
| 6 | C15487 | unpublished | 2L:23B* | 2L:28B(5e-134) |
| 7 | C01091 | unpublished | 2L:22C* | 2L:24C(2e-125) |
| 8 | C15834 | unpublished | 2L:18A* | 2L:24A(9e-133) |
| 9 | Ag1783 | AGAP001783 | 3R:31A | 2R:9B |
| 10 | Ag1763s | AGAP001763 | 3R:28C | 2R:9A |
| 11 | C11163 | unpublished | 3R:27A* | 2R:8B(3e-117) |
| 12 | C05257 | unpublished | 3L:38B | 3L:39C(2e-120) |
| 13 | Ag10402 | AGAP010402 | 3L:33C | 3L:38C |
The e-values are indicated in parentheses after the locations. Asterisk indicates major hybridization signal in An. sinensis.
Chromosomal arm homology was established by comparing the physical locations of probes in An. sinensis and An. gambiae. For comparison purposes, the chromosomal arms of An. gambiae were numbered from 1 to 5 to show homology as follows: X = 1; 2R = 2; 2L = 3; 3R = 4; 3L = 5 (Table 2). Our results indicate that chromosomes X, 2L, and 3L are homologous between the two species, while the 2R arm of An. sinensis corresponds to the 3R arm of An. gambiae and vice versa (Table 2). These results suggest a whole-arm translocation between the two species. Partial arm translocations or pericentric inversions are not commonly found in malaria mosquitoes and so our results are consistent with previous reports in Anopheles (Holt et al., 2002; Sharakhova et al., 2010; Xia et al., 2010; Sharakhova et al., 2011).
Table 2.
Correspondence between chromosomal arms among species of genus Anopheles
| X | 2L+2R | 3L+3R | |
|---|---|---|---|
| An. gambiae (subgenus Cellia) | 1 | 3+2 | 5+4 |
| An. funestus (subgenus Cellia) | 1 | 4+2 | 5+3 |
| An. stephensi (subgenus Cellia) | 1 | 5+2 | 3+4 |
| An. nili (subgenus Cellia) | 1 | 5+2 | 3+4 |
| An. albimanus (subgenus Nyssorhynchus) | 1 | 4+2 | 3+5 |
| An. sinensis (subgenus Anopheles) | 1 | 3+4 | 5+2 |
This table has been modified from Sharakhov and Sharakhova, 2008.
For comparison purposes, chromosomal arms are numbered from 1 to 5 to show homology as proposed by (Green and Hunt, 1980).
Identification of the 3Ra polymorphic inversion
One polymorphic paracentric chromosomal inversion was found on the 3R arm (Fig. 3). This inversion was first observed in the Shanghai strain of An. sinensis (Sun and Coluzzi, 1989). That study mapped both breakpoints to regions 26C and 30A. However, in the low-resolution image, the exact breakpoints could not be determined (Sun and Coluzzi, 1989). In our study, the distal and proximal breakpoints of the 3R+a arrangement were mapped to regions 28C and 31A, respectively. This mapping was based on the banding patterns of standard, inverted, and heterozygous arrangements (Fig. 1). Five closely located bands in subdivision 28C, four bands in subdivision 30B, and one light and one dark band in subdivisions 30B–C, can serve as landmarks to distinguish between the standard and inverted variants of inversion 3Ra (Fig. 1). In the case of the standard (3R+a/+a) chromosome, the banding pattern is arranged as four bands+one light band+one dark band in the proximal part of the chromosome, while in the inverted (3Ra/a) chromosome, the bands appear as one dark band + one light band + four bands near the distal part of the chromosome.
Fig. 3. The heterozygous polymorphic inversion on 3R arm of An. sinensis from Shanghai strain.
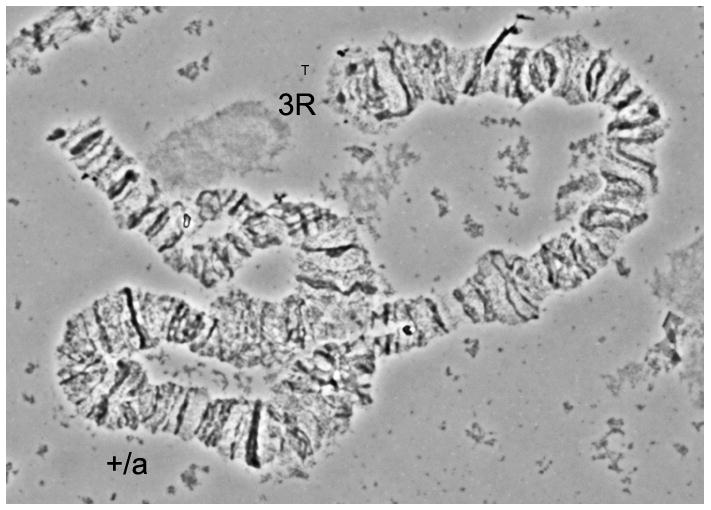
+/a indicates the heterozygous chromosome of 3R in An. sinensis, T indicates telomere.
Discussion
Anopheles sinensis is an important vector of P. vivax in southeast Asia. Several cytogenetic maps have been developed for various wild populations for comparative investigations of species differentiation (Kanda, 1968; Sharma, 1971; Li, et al., 1983; Ye, et al., 1983). However, so far, most published maps have been either drawn maps or photomaps made from low-resolution chromosomal images. In contrast to the previously drawn maps (Li, et al., 1983; Ye, et al., 1983), our standard photomap for An. sinensis is constructed from high-resolution chromosome images that provide more detail banding patterns. Moreover, we slightly modified some borders to make the photomap more suitable for genome mapping.
Our successful mapping of thirteen probes (Fig. 1 and Fig. 2) has shown the feasibility of using An. sinensis chromosomes for cytogenetic studies and genome mapping. We established the chromosome arm correspondence between An. sinensis and An. gambiae, demonstrating that chromosomes X, 2R, 2L, 3R and 3L of An. sinensis correspond to the X, 3R, 2L, 2R and 3L arms in An. gambiae. These results strongly suggest a whole-arm translocation of the autosomal arms between the two species (Table 2). This result agrees with the conclusion derived from previous mapping studies of the species from the subgenera Cellia and Anopheles (Cornel and Collins, 2000). Thus, our study has established a foundation for further comparative genomics studies of An. sinensis with other mosquitoes.
Inversion polymorphism has been extensively used for population genetic studies of malaria mosquitoes. Japanese cytogeneticist T. Kanda, who first described the polytene chromosomes of An. sinensis in 1968, initiated the analysis of genetic divergence in natural populations of An. sinensis (unpublished). He found that inversion 3Ra (28A-31B) existed with low frequency across Japan, while inversion 2Ra (12D-14B) was limited to only the Konosu population in Saitama Prefecture. Later, inversion 2Rb (9A-11D) was reported in the Engaru population of An. sinensis (Oguma, 1976). Due to the low resolution of images obtained from salivary gland chromosomes (Oguma, 1976), the breakpoints of these inversions could not be determined. Although there is a great deal of data on cytogenetic studies of An. sinensis, no inversion polymorphism has been examined in chinese populations. The only inversion on 3R described in the Shanghai laboratory strain was recorded in 1989 at the very low frequency of 4%; and the breakpoints were positioned in regions 26C and 30A (Sun and Coluzzi, 1989). In our study, the frequency of heterozygous chromosomes (3R+a/ a) reached 50% in 2012 and 86% in 2013. By using straightened high-resolution images of polytene chromosomes in this study, we were able to map the breakpoints of inversion 3Ra to subdivisions 28C and 31A (Fig. 1). Polymorphic inversions are not distributed randomly in An. gambiae; 18 of 31 polymorphic inversions are on the 2R arm (Coluzzi, et al.,2002). Interestingly, the only polymorphic inversion discovered so far in An. sinensis is located on 3R, which corresponds to 2R in An. gambiae. The new cytogenetic photomap will help future discoveries of novel polymorphic inversions and will facilitate studies of the population structure and ecological adaptation of An. sinensis.
Supplementary Material
T indicates telomeric end, C indicates centromeric end.
Acknowledgments
We thank Dr. Yidong Wu, Dr. Shuwen Wu and Dr. Yihua Yang, Department of Entomology, Nanjing Agricultural University, for their assistance. We thank Ashley Peery and Melissa Wade for editing the manuscript. We thank The Chinese Center for Disease Control and Prevention, Shanghai, China for providing An. sinensis colony. This work was supported by the National Natural Science Foundation of China grant 31301877 (to A.X) and by the National Institutes of Health grant 5R21AI094289 (to I.V.S).
References
- Coluzzi M, Sabatini A, et al. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298(5597):1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, Collins FH. Maintenance of chromosome arm integrity between two Anopheles mosquito subgenera. J Hered. 2000;91(5):364–370. doi: 10.1093/jhered/91.5.364. [DOI] [PubMed] [Google Scholar]
- Foley DH, Klein TA, et al. Geographic distribution and ecology of potential malaria vectors in the Republic of Korea. J Med Entomol. 2009;46(3):680–692. doi: 10.1603/033.046.0336. [DOI] [PubMed] [Google Scholar]
- Harbach RE. The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94(6):537–553. doi: 10.1079/ber2004321. [DOI] [PubMed] [Google Scholar]
- Hay SI, Sinka ME, et al. Developing global maps of the dominant anopheles vectors of human malaria. PLoS Med. 2010;7(2):e1000209. doi: 10.1371/journal.pmed.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298(5591):129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Kanda T. Studies on karyotypes of some Japanese mosquitoes. Jpn J Exp Med. 1968;38(1):37–46. [PubMed] [Google Scholar]
- Li BW, Liu D, et al. polytene chromosomes of the salivary gland of Anopheles sinensis: II. re-identification of the X chromosome and chromosome mapping for A. sinensis. Bull Hunan Medical College. 1983;8(3):260–265. [Google Scholar]
- Liu H, Lu HJ, et al. Japanese encephalitis virus in mosquitoes and swine in Yunnan province, China 2009–2010. Vector Borne Zoonotic Dis. 2013;13(1):41–49. doi: 10.1089/vbz.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yang M, et al. Population structure of the malaria vector Anopheles sinensis (Diptera: Culicidae) in China: two gene pools inferred by microsatellites. PLoS One. 2011;6(7):e22219. doi: 10.1371/journal.pone.0022219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma Y. Chromosomal polymorphism and salivary gland chromosomes of hybrides between strains of Anopheles sinensis (Diptera: Culicidae) Japan J Genetics. 1976;51(4):229–236. [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Brown TL, et al. Species composition, larval habitats, seasonal occurrence and distribution of potential malaria vectors and associated species of Anopheles (Diptera: Culicidae) from the Republic of Korea. Malar J. 2010;9:55. doi: 10.1186/1475-2875-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Iwakami M, et al. Habitats and distribution of Anopheles sinensis and associated Anopheles hyrcanus group in Japan. J Am Mosq Control Assoc. 2005;21(4):458–463. doi: 10.2987/8756-971X(2006)21[458:HADOAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, Serazin AC, et al. Inversions and gene order shuffling in Anopheles gambiae and An. funestus. Science. 2002;298(5591):182–185. doi: 10.1126/science.1076803. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, Sharakhova MV. Cytogenetic and physical mapping of mosquito genomes. In: JFV, Abbington LE, editors. Chromosome Mapping Research Developments. New York: Nova Science publishers, Inc; 2008. pp. 35–76. [Google Scholar]
- Sharakhova MV, Xia A, et al. Arm-specific dynamics of chromosome evolution in malaria mosquitoes. BMC Evol Biol. 2011;11:91. doi: 10.1186/1471-2148-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhova MV, Xia A, et al. A standard cytogenetic photomap for the mosquito Anopheles stephensi (Diptera: Culicidae): application for physical mapping. J Med Entomol. 2006;43(5):861–866. doi: 10.1603/0022-2585(2006)43[861:ascpft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sharakhova MV, Xia A, et al. A physical map for an Asian malaria mosquito, Anopheles stephensi. Am J Trop Med Hyg. 2010;83(5):1023–1027. doi: 10.4269/ajtmh.2010.10-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma OP. Salivary gland chromosomes of Anopheles hyycanus Var. sinensis. J Cyto Genet Congr. 1971;(Suppl):171–181. [Google Scholar]
- Sinka ME, Bangs MJ, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YC, Coluzzi M. A paracentric inversion in the salivary gland chromosome 3R of Anopheles sinensis. Chinese journal of parasitology & parasitic diseases. 1989;7(4):288–291. [PubMed] [Google Scholar]
- Xia A, Sharakhova MV, et al. Genome landscape and evolutionary plasticity of chromosomes in malaria mosquitoes. PLoS One. 2010;5(5):e10592. doi: 10.1371/journal.pone.0010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SB, Tan JX, et al. A comparative study of the polytene chromosomes of Anopheles anthropophagus and Anopheles sinensis. Hereditas. 1990;12(5):17–18. [Google Scholar]
- Xu SB, Tan JX, et al. A comparative study of the chromosomes of Anopheles hyrcanus and Anopheles sinensis. Acta Entomologica Sinica. 1991;34(3):380–382. [Google Scholar]
- Xu XF, Wang ZW, et al. The salivary gland chromosomes of Anopheles sinensis Wied. from Zhengzhou. Entomotaxonomia. 1981;3(4):281–285. [Google Scholar]
- Ye B, Huang P, et al. The salivary gland chromosomes of Anopheles sinensis. Acta Genetica Sinica. 1981;8(1):42–49. [Google Scholar]
- Ye BH, Huang PQ, et al. Further studies on the salivary gland chromosomes of Anopheles sinensis. Acta Genetica Sinica. 1983;10(6):489–492. [Google Scholar]
- Ye BH, Shen SB, et al. A comparison of salivary chromosome and isozymes of three zymes of Anopheles sinensis in Xuzhou and Yixing districts. Acta Genetica Sinica. 1985;12(4):289–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T indicates telomeric end, C indicates centromeric end.