Figure 1.
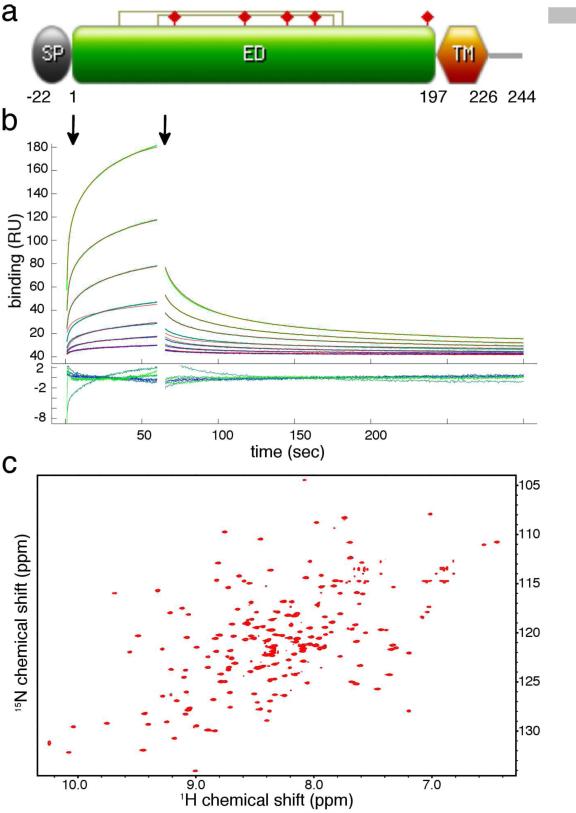
Biophysical characterization of m04ED for structural studies. (a) Domain organization of full-length m04/gp34, indicating the positions of signal peptide (SP), extracellular domain (ED) and transmembrane domain (TM). Disulfide bonds are indicated with connected lines, and glycosylation sites with filled red prisms. (b) SPR binding sensograms collected using immobilized m04ED (WT) with increasing concentrations of H2-Dd flow-through, as outlined in Experimental Procedures. The start of the injection (association) and wash out (dissociation) phases are indicated with vertical arrows. The data were fit using EVILFIT (Svitel et al., 2003) (thin red lines – Kd ~395μM). Residual errors in the fit are shown in the inset. As a negative control the MHC-I-like molecule MULT-1 was injected over the same SPR surface (Figure S1). (c) TROSY-HSQC 1H-15N correlation spectra of m04ED −C7S recorded at 900 MHz, 12 °C, pH 6.5.