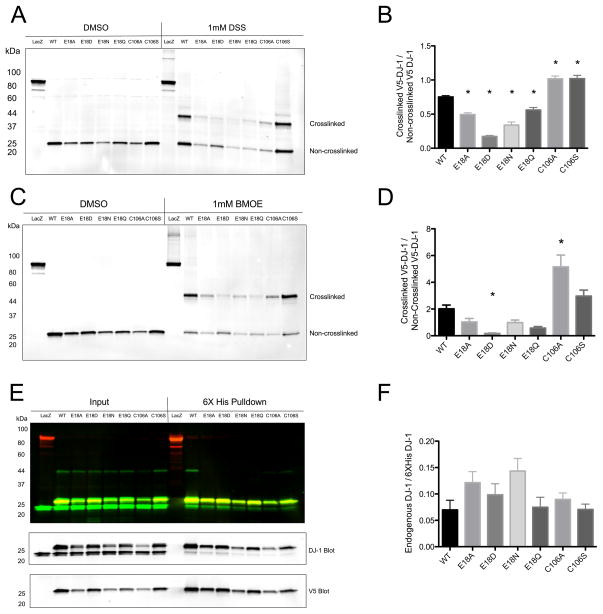
Figure 8.
DJ-1 crosslinking and heterodimer formation in HEK293FT cells. In Panel A, HEK293FT cells transiently transfected with 6xHis-V5 tagged wild type DJ-1 and mutants were exposed to the amine crosslinker DSS and V5-tagged protein was analyzed by Western blot. Crosslinked dimers of ~45 kDa are apparent for all proteins, but are less prominent for the Glu18 mutants. In Panel B, DSS crosslinking efficiency was quantified using densitometry (n=6 independent samples, * denotes p < 0.05 versus WT, one-way ANOVA followed by Bonferroni’s multiple comparison test). Panel C shows V5 Western blot analysis of proteins from HEK293FT cells transiently transfected with 6xHis-V5 tagged proteins and exposed to the sulfhydryl crosslinker BMOE. Crosslinked dimeric species for all DJ-1 proteins are evident at ~45 kDa. In Panel D, the crosslinking efficiency of BMOE was quantified using densitometry (n=9, * denotes p < 0.05 versus WT, one-way ANOVA followed by Bonferroni’s multiple comparison test). In Panel E, V5-6xHis tagged DJ-1 proteins were purified from HEK293FT lysates using Ni2+ affinity resin and then Western blots were probed with anti-V5 (red) and anti-DJ-1 (green) antibodies to examine heterodimer formation. In Panel F, the amounts of endogenous wildtype DJ-1 that co-purified with tagged exogenous proteins are quantified (n=6, one-way ANOVA p = 0.0546). All V5-6xHis-DJ-1 proteins are able to form mixed heterodimers with endogenous DJ-1 with comparable efficiency.