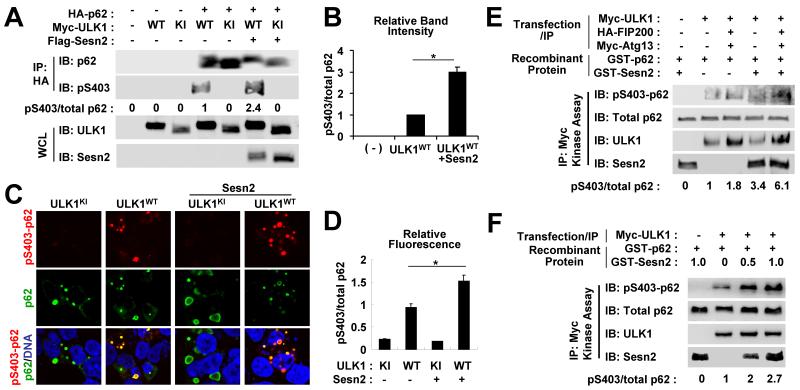
Fig. 6. Sestrin2 promotes ULK1-mediated phosphorylation of p62.
(A,B) HEK293 cells were transfected with plasmids expressing indicated proteins. Anti-HA immunocomplexes (IP) and whole cell lysates (WCL) were subjected to immunoblotting (IB). Relative phosphorylation of p62 at Ser403 (pS403/total p62) was quantified by densitometry and presented as numbers below each lane (A) and as a graph (B, n = 3). (C,D) HEK293 cells were transfected with plasmids expressing indicated proteins and immunostained with anti-phospho Ser403 p62 (red) and anti-total p62 (green) antibodies and DAPI (DNA, blue). Relative fluorescence of phosphorylated and total p62 in each aggregate was quantified and presented as a graph (n ≥ 20). (E,F) HEK293 cells were transfected with Myc-tagged ULK1 and its regulatory subunits FIP200 and Atg13. ULK1 kinase activity of anti-Myc immunocomplexes was assayed using recombinant GST-p62 protein. GST-Sesn2 protein (0.5 μg or as indicated) was directly added to the kinase reaction mixture. Amounts of phosphorylated and total proteins were assayed through immunoblotting. Relative phosphorylation of p62 at Ser403 (pS403/total p62) was quantified by densitometry and presented as numbers below each lane. *P < 0.05.