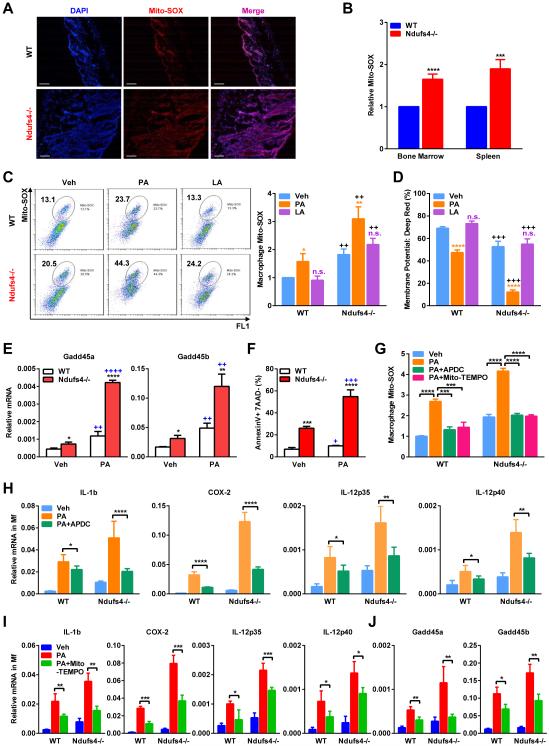
Figure 4. ROS level is elevated in Ndufs4−/− macrophages and exacerbated by fatty acids.
(A-B) Representative images of Mito-SOX staining of skin on P22. Scale bars, 25 μm.
(B) FACS quantification of Mito-SOX levels in bone marrow and spleen on P22 (n=5).
(C) Mito-SOX levels in BM-Mfs treated with PA or LA (400 μM). Left, FACS 2D dot plots. Right, Quantification (n=5).
(D) Mitochondrial membrane potential was decreased in Ndufs4−/− macrophages and further reduced by PA, but not LA (n=5), quantified by percentage of MitoTracker Deep Red via FACS. C-D, * and n.s. compare treatment with vehicle control in the same genotype; + compares Ndufs4−/− with WT in the same treatment group.
(E-F) Expression of the stress sensor genes Gadd45α/β (E) and apoptosis (F) were higher in Ndufs4−/− macrophages and further potentiated by PA (n=3). * compares Ndufs4−/− with WT under the same treatment; + compares PA with vehicle in the same genotype.
(G) PA induction of mitochondrial ROS in macrophages, measured by Mito-SOX, was abolished by ROS inhibitors 2R,4R-APDC (50 μM) or Mito-TEMPO (50 μM).
(H-I) ROS inhibitors 2R,4R-APDC (H) or Mito-TEMPO (I) partially rescued the PA-activated inflammatory gene expression in WT and Ndufs4−/− macrophages. (J) Mito-TEMPO partially rescued the PA-activated stress sensor gene expression in WT and Ndufs4−/− macrophages. BM-Mfs were treated with PA (400 μM), with or without APDC or Mito-TEMPO (50 μM). Error bars, SD.