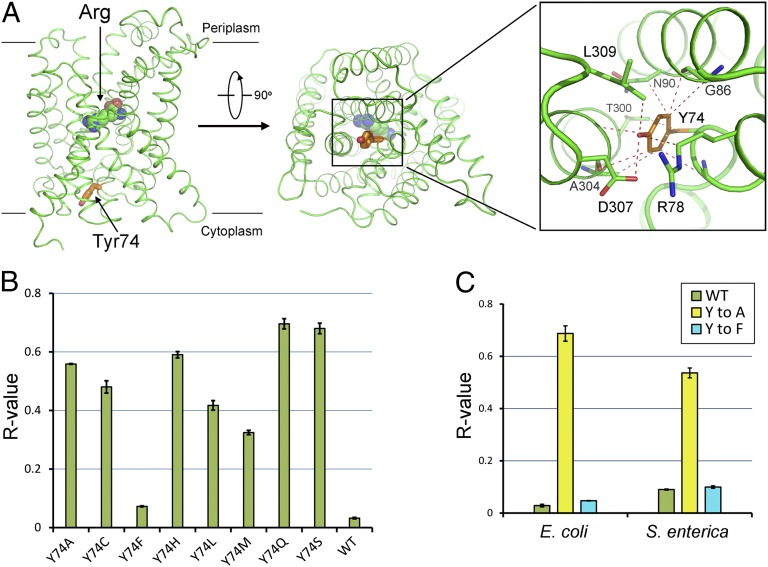
Fig. 4.
Proposed mechanism of pH-sensing by Tyr74. (A) Tyr74 blocks the transport path by interacting with various surrounding residues in AdiC. Tyr74 (orange) serves as a gate at the cytosolic side of AdiC. Van der Waals contacts within 4 Å are represented by red dashed lines. The bound Arg is shown in spheres. (B) Replacement of Tyr74 by Phe, but not by any other amino acid, resulted in abrogation of pH-dependent substrate transport. The R values for eight variants are compared with that for the WT AdiC protein. (C) Tyr74 likely serves as the pH sensor in AdiC from S. enterica. The R value for WT AdiC from S. enterica is similar to that for the Y74F variant. In contrast, the R value for the Y74A variant from S. enterica is ∼0.54, sixfold higher than that for WT AdiC from S. enterica.