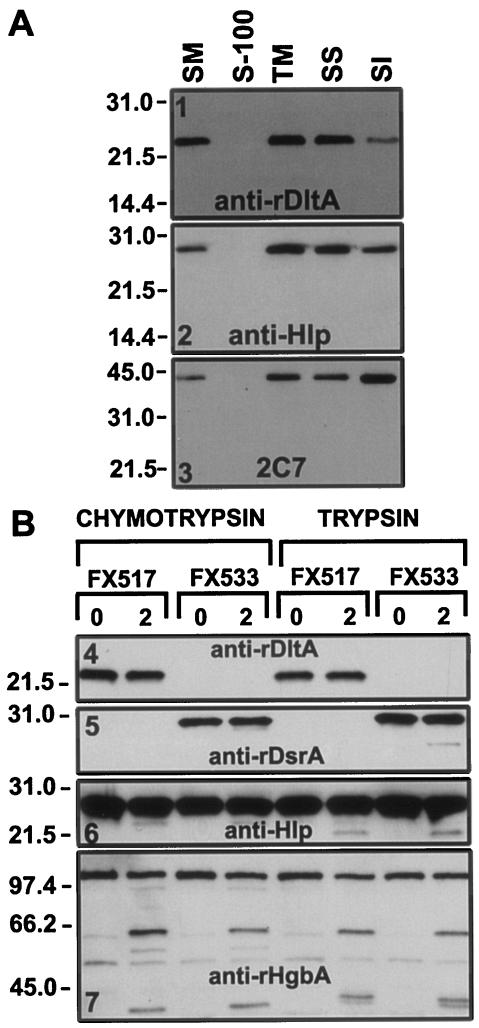
FIG. 7.
Cell fractionation and protease cleavage of H. ducreyi. (A) Cell fractionation. Whole cells of H. ducreyi strain 35000HP were subjected to detergent fractionation as described in the Materials and Methods. Then, 10 μg of each preparation was subjected to SDS-PAGE and Western blotting with anti-rDltA, anti-Hlp, and 2C7 antibodies as primary probes and with anti-rabbit-AP or anti-mouse-AP (2C7) as secondary probes. The blots were developed by using chemiluminescence. Lanes: SM, starting material; S-100, supernatant from a centrifugation at 100,000 × g; TM, total membranes; SS, SS supernatant; SI, SI pellet. (B) Protease cleavage. H. ducreyi cells from strains FX517 and FX533 (ca. 2 × 108 CFU/ml) were subjected to protease cleavage with either chymotrypsin (5 μg/ml) or trypsin (20 μg/ml) for 2 min at 35°C. Cells were pelleted, solubilized in Laemmli sample buffer, and subjected to SDS-PAGE (∼107 CFU/lane) in reducing conditions and Western blotting. Lanes: 0, before protease treatment; 2, after 2 min of protease treatment.