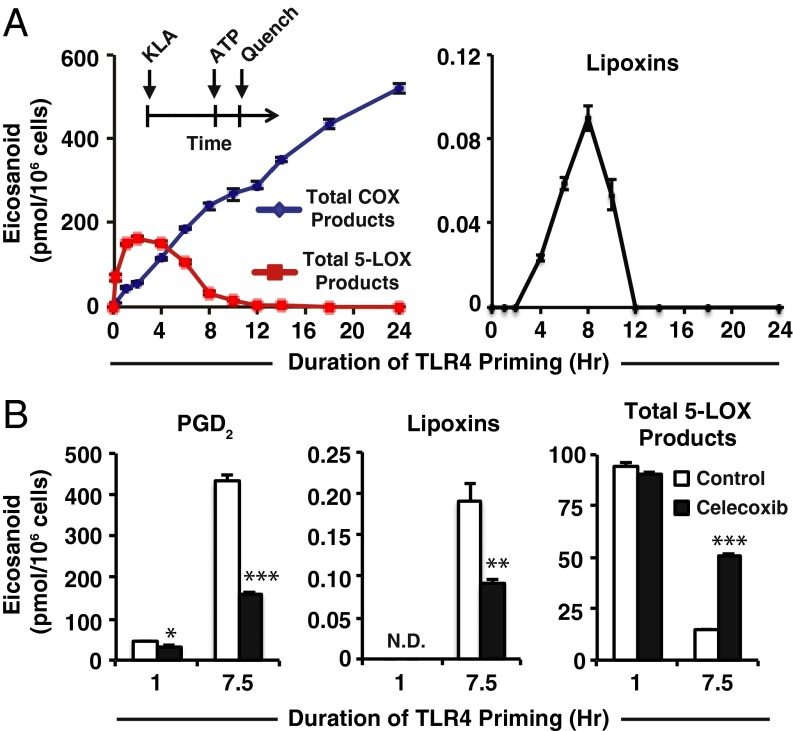
Fig. 1.
Duration of TLR4 priming controls purinergic 5-LOX product formation and lipoxin biosynthesis. (A, Inset) Protocol for TLR4 priming (Kdo2 lipid A, KLA) starting at time = 0 followed by ATP stimulation at indicated times and subsequent reaction quench as endpoint (further details can be found in SI Materials and Methods); eicosanoid levels from RAW264.7 (RAW) cell medium after TLR4 priming with 100 ng/mL KLA for varying durations before stimulation with 2 mM ATP for the final 10 min include total COX products (PGD2, PGE2, PGF2α, PGJ2, 15-deoxy PGD2, 15-deoxy PGJ2, 11-HETE, and 15-HETE); total 5-LOX products (5-HETE, LTC4, 11-trans LTC4, LTB4, 6-trans,12-epi LTB4, 6-trans LTB4, and 12-epi LTB4); lipoxins (LXA4 and 15-epi–LXA4). (B) Levels of PGD2, lipoxins (LXA4 and 15-epi–LXA4), and total 5-LOX products (as in A) from RAW medium after KLA priming for the indicated times in the absence (white bars) or presence (black bars) of 50 nM celecoxib (∼IC50) followed by stimulation with ATP for the final 30 min; PGD2 levels were decreased with celecoxib treatment vs. control with 1-h TLR4 priming (*P < 0.01) and 7.5 h TLR4 priming (***P < 0.0001); lipoxin levels were not detected (N.D.) with 1 h priming and were decreased at 7.5 h TLR4 priming with celecoxib treatment vs. control (**P < 0.005); total 5-LOX products with 1-h priming were not significantly different with celecoxib vs. control, and at 7.5-h priming were increased with celecoxib vs. control (***P < 0.0001). Data are mean values of three separate experiments ± SEM.