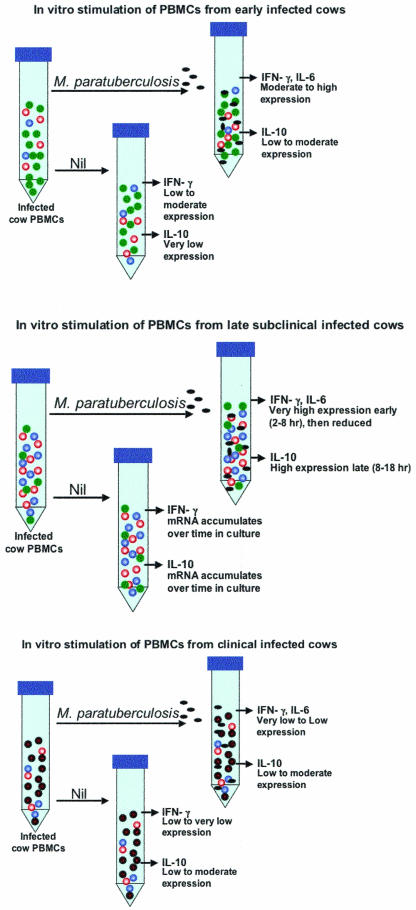
FIG. 2.
Proposed in vitro responses of PBMCs from progressively infected cows to stimulation with M. avium subsp. paratuberculosis. (A) Removal of peripheral immune cells from early-stage infected cows and stimulation in vitro with M. avium subsp. paratuberculosis results in a minor population of antigen-reactive proinflammatory T cells (red circles) producing significant amounts of IFN-γ. Expression of IL-6 and IL-1α is also upregulated in these proinflammatory cells. A small number of suppressor T cells (blue circles) may also be present in PBMCs from cattle in early stages of infection, but in numbers insufficient to control the proinflammatory response. Many peripheral immune cells are present in PBMC preparations that are not reactive to M. avium subsp. paratuberculosis antigens (green circles). Thus, the predominant response to in vitro stimulation of PBMCs from cattle at this stage of infection is production of IFN-γ, IL-6, and IL-1α.(B) PBMCs during the late subclinical stage of infection contain high numbers of M. avium subsp. paratuberculosis-reactive proinflammatory cells (red circles) that are already activated and produce significant amounts of IFN-γ and IL-6, even without further antigen stimulation in vitro (Nil). In vitro, production of IFN-γ, IL-6, and perhaps IL-1α is rapidly enhanced by introduction of M. avium subsp. paratuberculosis antigens, although this response is eventually quelled by IL-10 produced from large numbers of activated suppressor cells (blue circles). Overall, the percentage of non-M. avium subsp. paratuberculosis-reactive cells (green circles) has been reduced, although the balance of CD4+, CD8+, and γδ TCR+ T cells has not changed appreciably. (C) Due to the presence of large numbers of cytotoxic suppressor cells and reduced numbers of proinflammatory cells, PBMCs extracted during clinical stages of infection produce less IFN-γ and IL-6 in response to M. avium subsp. paratuberculosis antigens than cells from late subclinically infected cattle. Loss of IL-10-producing suppressor cells, which are present in large numbers during subclinical infection, also leads to lower production of this cytokine in PBMCs from clinically infected cattle. Prolonged in vitro stimulation (>16 h) of PBMCs from clinically infected cattle with M. avium subsp. paratuberculosis antigens results in apoptosis of many proinflammatory and cytotoxic suppressor cells. PBMCs from cattle in clinical stages of Johne's disease thus produce little IFN-γ, IL-6, and IL-1α in response to M. avium subsp. paratuberculosis antigens, although continued proliferation of the cytotoxic cell population has caused a profound change in the pattern of gene expression observed in PBMCs from these animals relative to those from uninfected cattle or cattle in earlier stages of infection. Although not shown in this figure, the predominant remaining response to M. avium subsp. paratuberculosis in clinically infected cattle is production of IgG1 from antigen-stimulated B cells. The proposed cytotoxic cell population may also kill non-M. avium subsp. paratuberculosis-reactive T cells in vitro (and perhaps in vivo), thus leading to an observed general immune cell anergy in PBMCs from clinically infected cattle.