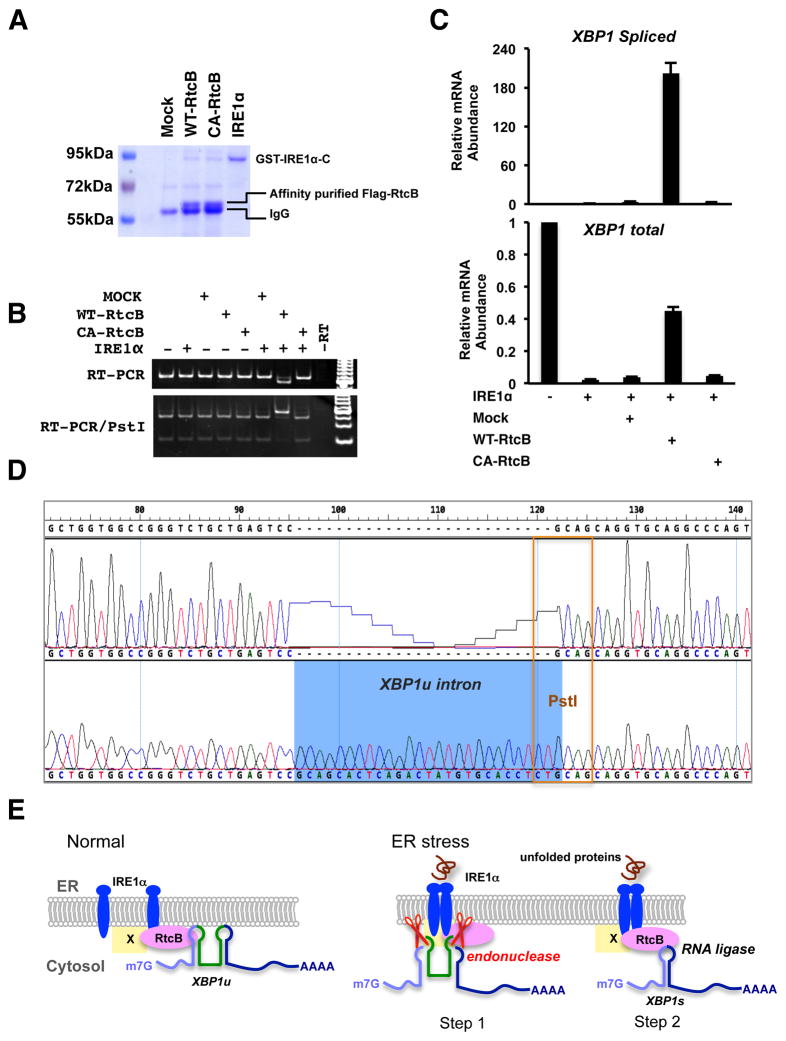
Figure 7. IRE1α and RtcB reconstitute XBP1 splicing in vitro.
A. A Coomassie blue stained gel showing the affinity-purified RtcB proteins and the recombinant cytoplasmic domain of IRE1α. Both Flag-RtcBWT and Flag-RtcBC122A proteins were purified from the HEK293T stable cell lines described in Fig. 6A. B. An XBP1u intron-containing RNA substrate described in Fig. S7 was generated by in vitro transcription. Indicated purified proteins were incubated with the RNA substrate. The RNA products were purified and analyzed by RT-PCR. Note that the PCR was carried at saturation to increase signal intensity. The size shift and Pst I digestion patterns showed that wild type RtcB and IRE1α were both required and sufficient for XBP1u splicing in vitro. C. RT-qPCR analysis of the in vitroXBP1 splicing. D. Sequence analysis of the PCR products confirmed that the reconstituted splicing reaction occurred at the exact exon-intron junction. E. A working model to demonstrate the role of RtcB during mammalian UPR. Under normal condition, a subset of RtcB is in complex with IRE1α while binding to XBP1u mRNA. Upon ER stress, IRE1α oligomerizes, trans-autophosphorylates, and activates its endonuclease activity to excise a 26-nt intron of XBP1u. The IRE1α-associated RtcB then ligates two XBP1 exons to generate XBP1s mRNA. By physical interaction between RtcB and IRE1α, the two sequential steps of unconventional XBP1 splicing occur on the ER membrane. At present, it is not known whether RtcB directly interacts with IRE1α or through an intermediate (marked as “X”).