Abstract
Cellular receptors for KSHV attachment and entry were characterized using tyramide signal amplification (TSA)-enhanced confocal microscopy. Integrins αVβ3, αVβ5 and α3β1 were detected on essentially all the actin-based cell surface microdomains that initially bind KSHV, while the presence of CD98 and heparan sulfate (HS), the putative attachment receptor, was more variable. KSHV bound to the same cell surface microdomains with and without HS indicating that initial attachment of KSHV is not dependent on HS and that receptors other than HS can mediate attachment. A human salivary gland (HSG) epithelial line was identified, which lacks αVβ3 but expresses high levels of HS, α3β1 and other putative KSHV receptors. These cells were resistant to KSHV-binding and infection. Reconstitution of cell surface αVβ3 rendered HSG cells highly susceptible to KSHV infection, demonstrating a critical role for αVβ3 in the binding and entry of KSHV that is not shared with other proposed receptors.
Keywords: KSHV, Kaposi’s sarcoma-associated herpesvirus, virus attachment, virus entry, virus binding, tyramide signal amplification, integrin αVβ3, cell surface microdomains
INTRODUCTION
The binding of viruses to specific plasma membrane domains stimulates particle movements, receptor clustering, and protein signaling and activation, all of which ultimately triggers uptake of virus by endocytosis or direct transmission of virus to neighboring cells [1]. Plasma membrane domains containing viral receptors are often specialized actin-based structures generated by distinct filament assemblies, including sheet-like protrusions (lamellipodia and ruffled membranes) with branched actin filaments and finger-like protrusions (filopodia and microvilli) that contain parallel filament bundles [2]. Lamellipodia and filopodia adhere cells to the substratum and make contact with adjacent cells, while ruffled membranes and microvilli are non adherent “free” structures on the apical cell surface.
Particle tracking studies have shown that viruses, such as murine leukemia virus, human immunodeficiency virus, avian leukemia virus [3,4], human adenovirus [5,6], herpes simplex virus type-1 (HSV-1) [7], and human papillomavirus type 16 [8] move on filopodia and utilize actin/myosin II driven retrograde flow as a means of virion delivery to sites of particle uptake at connecting lamellipodial edges. Similarly, the spread of viruses from infected to non infected cells involves virus transport along filopodial bridges contacting adjacent target cells [3,9-11]. In some cases, virus attachment to filopodia is mediated by plasma membrane heparan sulfate (HS) glycosaminoglycans [7,8,12].
HSV-1 has been shown to bind preferentially to filopodia and not the cell body as a result of restricted distribution of HS on filopodial microdomains [7]. In this case, HSV-1 attachment involved the interaction of envelope glycoprotein B with HS, while cell infection required the transport of HSV-1 along filopodia toward the cell body where viral glycoprotein D was recognized by a second cell surface entry receptor [7]. Other herpesviruses, such as cytomegalovirus [13], varicella zoster [14], and Kaposi’s sarcoma-associated herpesvirus (KSHV) [15] have envelope glycoproteins with HS recognition motifs, however, it is not known if HS binding targets the viruses to specific membrane microdomains to initiate cell infection.
KSHV is the causative agent of Kaposi’s sarcoma, pleural effusion lymphoma and multicentric Castleman’s disease [16]. Several KSHV virion proteins bind HS, including glycoprotein B [15], K8.1 [17,18], glycoprotein H [19] and complement control protein (KCP) [20]. Similar to HSV-1, KSHV virions may utilize actin retrograde flow to move to membrane domains containing secondary entry receptors, since perturbation of the actin cytoskeleton causes a reduction in KSHV infection [21-23]. Several cell surface proteins have been implicated as KSHV entry receptors, including DC SIGN [24,25], the cystine transporter (xCT) light chain [26] - CD98 heavy chain heterodimer [27], the ephrin receptor tyrosine kinase A2 (EphA2) [28], syndecan [19,28], and the integrins α3β1, αVβ3 and αVβ5 [27,29,30].
We have shown that the αVβ3 integrin binds specifically to an “RGD” motif at the N-terminus of the KSHV envelope glycoprotein B [30]. The HS binding domain of KSHV glycoprotein B is positioned downstream of the RGD motif [31], suggesting that these motifs may cooperate in virus attachment and entry through cell surface domains containing both HS and αVβ3 integrin. However the distribution of HS and αVβ3 integrin relative to cell surface domains binding KSHV has not been established. Although KSHV infection is inhibited by enzymatic removal of cell surface HS, the inhibition is incomplete as nearly 40% of the cells still become infected at the highest enzyme concentrations tested [31]. This raises the possibility that KSHV attachment may not be dependent on HS interactions alone.
We recently identified cell surface structures initiating attachment of KSHV to target cells using gradient purified DNP hapten labeled KSHV virions [32]. An ultra sensitive fluorescent enhancement using tyramide signal amplification (TSA) was developed to visualize DNP-KSHV bound to HT1080 fibrosarcoma cells, which are highly sensitive to KSHV infection [30]. We determined that KSHV binds to specific cellular microdomains, including actin-based filopodia, lamellipodia, ruffled membranes, microvilli and intercellular junctions. KSHV-binding domains were also identified on the dorsal cell surface and on a distinct supranuclear domain. Quantitation of bound virus revealed a significant increase on mitotic cells. Importantly, the binding of KSHV to the cell surface domains was inhibited by heparin treatment, confirming previous studies [31]. In the current study, we determined that essentially all of the cell surface domains binding KSHV contain the integrins αVβ3, αVβ5 and α3β1, which colocalized with bound DNP-KSHV. We determined that KSHV binds equally well to microdomains with and without cell surface HS, suggesting that receptors other than HS can function in virus attachment. Furthermore, a human salivary gland epithelial cell line that highly expresses all of the putative non lymphoid KSHV receptor proteins except αVβ3 was resistant to KSHV infection until cell surface αVβ3 was reconstituted by transient expression of β3 integrin. Our findings provide further support for a critical role of αVβ3 integrin in virus binding and entry that is not shared by the other putative KSHV receptors, including HS, integrins α3β1 and αVβ5, EphA2 and xCT/CD98.
RESULTS
KSHV colocalizes with αVβ3 integrin on cell surface binding domains
Using DNP-labeled gradient purified KSHV virions and ultrasensitive TSA fluorescence amplification, we have previously identified KSHV attachment sites on the surface of highly infectable HT1080 fibrosarcoma cells [32]. The DNP-labeled KSHV was highly infectious, maintained expression of envelope associated glycoproteins and showed minimal evidence of aggregation. The HT1080 cells were fixed with paraformaldehyde to prevent virus induced receptor modulation, thus providing a system to capture the initial interactions of the virions with existing cell surface binding sites. These studies revealed that TSA-enhanced fluorescence was 500 fold more sensitive than typical FITC-based techniques, enabling a detailed and accurate localization of DNP-labeled KSHV on the cell surface.
Previously, we also showed that KSHV virions bind specifically to affinity-purified αVβ3 integrin heterodimer in the absence of other cellular proteins [30]. We therefore examined whether the KSHV cell surface binding domains contain αVβ3 using gradient purified DNP-KSHV virions and monoclonal antibodies recognizing the β3 integrin with confocal fluorescence microscopy. Furthermore, we developed a technique for sequential TSA fluorescence enhancement to allow simultaneous detection of antibodies reacting with DNP-KSHV and αVβ3 using tyramides coupled to different Alexa fluors.
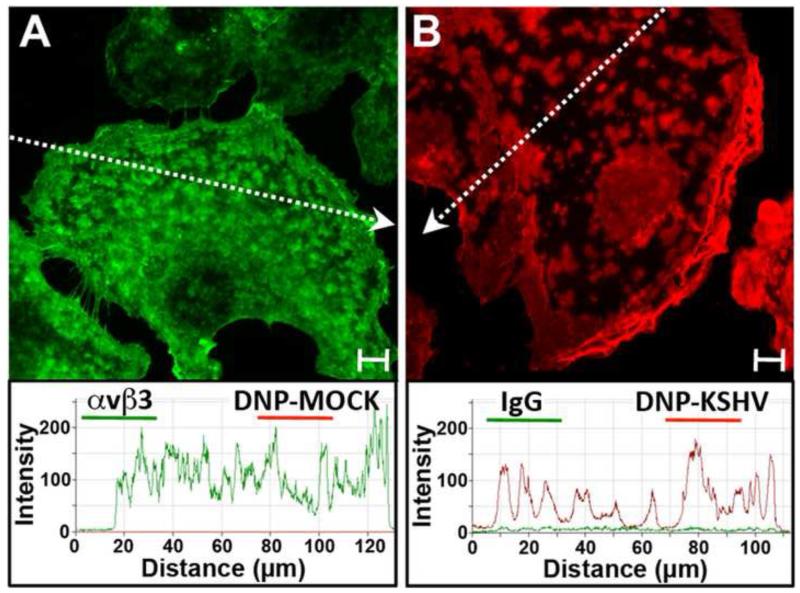
Initially, we tested the specificity of sequential TSA enhancement steps using cells incubated with DNP-mock virus control and mouse anti-β3 integrin or cells incubated with DNP-KSHV and non-immune mouse IgG control. First, HT1080 cells were fixed with paraformaldehyde and incubated with a mixture of mouse anti-β3 antibody and DNP-mock virus control, as described in Materials and Methods. The cells were washed, fixed again, and incubated with goat anti-mouse IgG G20 gold conjugate followed by HRP-coupled donkey anti-goat IgG and TSA 488. Due to the particle size, the 20 nm gold conjugate is excluded from the intracellular compartment and only cell surface bound anti-β3 antibody is detected via the TSA 488 fluorescence. Residual peroxidase activity was blocked with 1% H2O2. Non-specific binding of DNP from the mock virus control was evaluated using monoclonal rat anti-DNP followed by goat anti-rat IgG coupled to HRP and TSA 647. Strong TSA 488 enhanced β3 integrin staining was detected on specific cell surface domains (Fig. 1A). No TSA 647 signal was observed from the mock virus control. The histogram shows the quantitation of detected fluorescence along the dotted arrow.
Figure 1. Specificity of DNP-KSHV cell binding and two-color sequential TSA enhancement.
HT1080 cells were fixed and incubated with A) mouse anti-β3 integrin antibody and control DNP-mock gradient fraction or B) control non-immune mouse immunoglobulin (Ig) and DNP-KSHV gradient fraction. Bound proteins were detected with anti-mouse IgG-HRP and TSA 488 (green) followed by rat anti-DNP, anti-rat-HRP, and TSA 647 (red). The histogram shows the quantitation of detected fluorescence along the dotted arrow segment. Bar=10μm
The converse control experiment was performed using DNP-KSHV and non-immune mouse IgG following the same sequential TSA enhancement protocol described above. Strong staining of DNP-KSHV was detected on distinct cell surface domains, including intercellular junctions, isolated microdomains on the dorsal cell surface and a supranuclear domain (Fig. 1B), as shown previously [32]. No TSA 488 signal was observed from the non-immune mouse IgG control (Fig. 1B). Thus, these control experiments showed no evidence of antibody cross-reactions of the species specific HRP-labeled secondary antibodies to the mouse and rat primary antibodies, nor was there non-specific binding of the non-immune mouse IgG or artifactual DNP-labeled material within the mock virus control. Finally, no artifactual enhancement of the reactants in the primary TSA step was induced by the secondary TSA step.
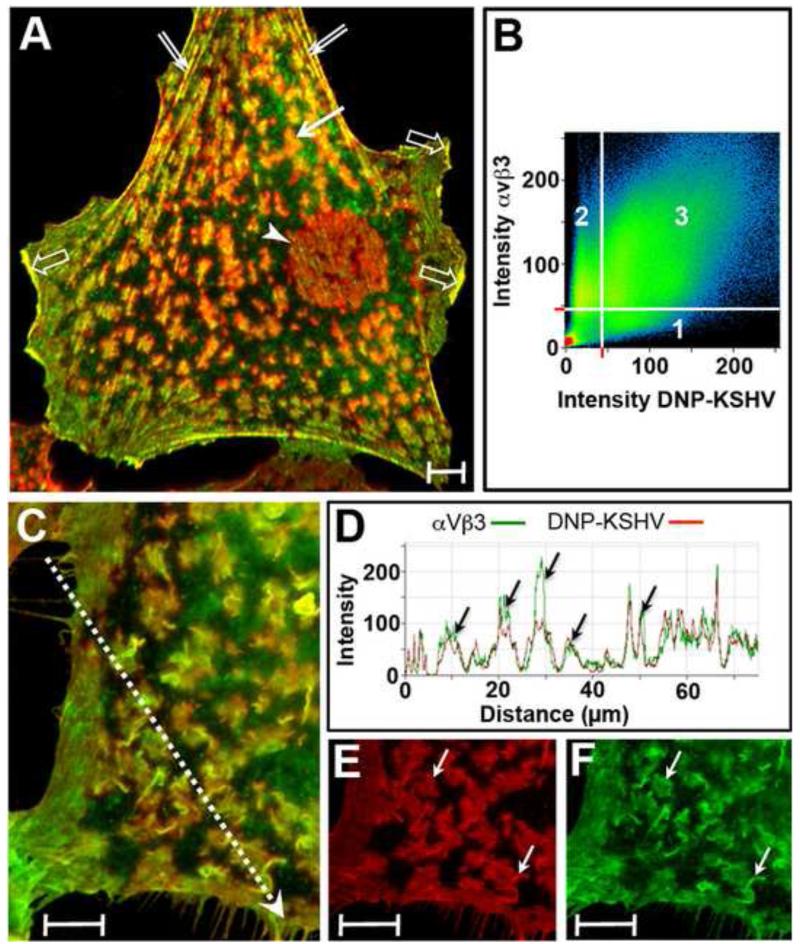
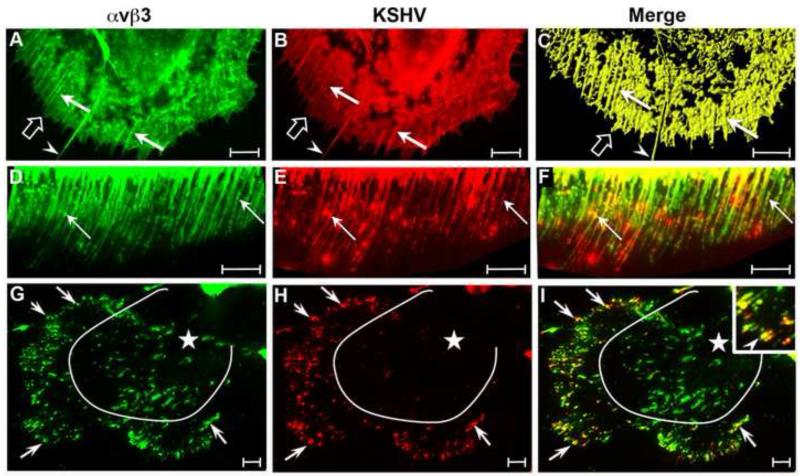
To determine whether DNP-KSHV binds to the cell surface domains containing αVβ3, HT1080 cells were fixed, incubated with both DNP-KSHV and mouse anti-β3 integrin antibody, using the sequential TSA enhancement steps described above. An overview micrograph (Fig. 2A) of the confocal analysis of the β3 integrin (green) and bound DNP-KSHV (red) fluorescence revealed a high level of colocalized pixels (yellow/orange) on specific cellular domains, including actin-based fibrillar structures (double arrow), lamellipodia and ruffled membranes (open arrows). In addition, colocalized pixels were detected in isolated microdomains on the dorsal cell surface (solid arrow) and in a distinct supranuclear domain (arrowhead)(Fig. 2A). A scatter diagram (Fig. 2B) showing the distribution of KSHV-only (scatter region 1), αVβ3-only (scatter region 2) and colocalized pixels (scatter region 3) revealed a high degree of colocalization of KSHV and αVβ3 in Figure 2A. Manders colocalization coefficients of M1 = 0.88 and M2 = 0.79, were determined for the red and green fluorescence on the dorsal cell surface, where M1 indicates the fraction of DNP-KSHV detected that overlaps with αVβ3 (M1 = KSHV/αVβ3) and M2 indicates the fraction of αVβ3 detected that overlaps with DNP-KSHV (M2 = αVβ3/KSHV). These results are representative of the other cells in the analysis. Thus, nearly 90% of KSHV attachment sites on HT1080 cells contained αVβ3 integrin, while the majority (80%) of membrane domains containing αVβ3 integrin bound KSHV.
Figure 2. Colocalization of KSHV-binding sites and αVβ3 integrin on cell surface membrane structures.
A) HT1080 cells were fixed and incubated with anti-β3 integrin antibody and DNP-KSHV and the fluorescent signals were sequentially enhanced with TSA 488 (anti-β3, green) and TSA 647 (anti-DNP, red). Colocalized β3 integrin and bound KSHV appears yellow/orange. Manders overlap coefficients M1 and M2, determined by the Zeiss LSM Pascal software, were 0.88 and 0.79, respectively, where M1 indicates the percentage of DNP-KSHV detected that overlaps with αVβ3, and M2 indicates the percentage of the αVβ3 detected that overlaps with DNP-KSHV. B) A scattergram of the αVβ3 (green) and KSHV (red) pixels in panel A. C) The dorsal surface of another cell was analyzed at a higher magnification to measure fluorescent intensities for β3 integrin (green) and bound KSHV (red) along the dashed arrow. D) Histogram of the fluorescent intensities along the arrow in (C). Topography of bound KSHV (E) is shown compared to αVβ3 integrin (F). The merged image of this cell is shown in panel C. Bar = 10μm
Colocalization on the cell surface microdomains was examined more closely at higher magnification near the cell margin of a different cell (Fig. 2C). The intensity profiles for each fluorescent signal was determined along the line segment (dotted arrow). A close overlap of the signal profiles was detected, demonstrating a high degree of colocalization of bound KSHV (red) and αVβ3 (green) (Fig. 2D). Moreover, membrane areas devoid of αVβ3 integrin had no bound virus. These results are representative of other regions of the cell. Examining KSHV and αVβ3 signals individually in this region confirmed that the bound KSHV (red) and cell surface αVβ3 integrin (green) were present on morphologically similar domains (Fig. 2E and F, respectively; see arrows). These results strongly support the specificity of the binding of the DNP-labeled KSHV to the distinct cell surface microdomains, as DNP-KSHV binds primarily to sites containing αVβ3 integrin, a putative KSHV entry receptor [30].
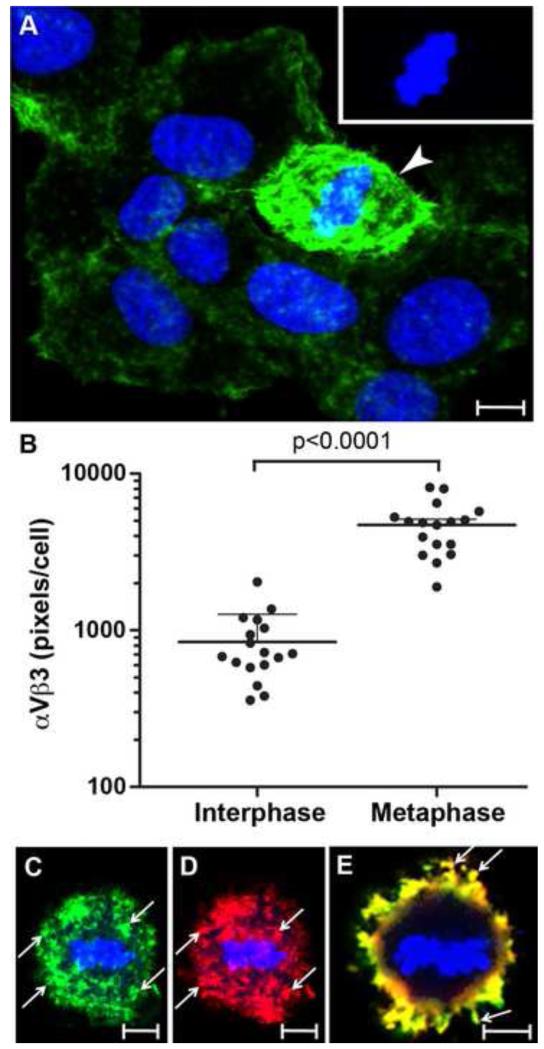
We have previously shown that the binding of DNP-KSHV to mitotic cells was nearly six-fold higher than the binding to interphase cells [32]. Since studies on rat fibroblasts showed that αVβ3 expression was upregulated on mitotic cells [33], we examined the αVβ3 expression levels on mitotic HT1080 cells with obvious metaphase chromosomes, using immunoconfocal microscopy with the mouse anti-β3 integrin antibody and TSA enhancement, as described above (see Fig. 3A). Although the number of mitotic cells in the culture was limited, 42 cells with metaphase chromosomes were identified in micrographs containing 413 non-mitotic cells. Quantitation of the cell surface αVβ3 integrin pixels (intensity range 50-250) yielded an average of 851 ± 420 pixels/cell associated with interphase cells and 4527 ± 1531 pixels/cell associated with mitotic cells (Fig. 3B). Furthermore, the mean pixel intensity in the mitotic cells was 152 compared to 72 in the interphase cells. Factoring in the pixel intensity differences, our results indicate that mitotic HT1080 cells have more than 10 fold higher levels of cell surface αVβ3 expression than interphase cells (P<0.0001), confirming previous studies on rat fibroblasts [33]. Using the sequential TSA enhancement steps described above, the distribution of αVβ3 and bound DNP-KSHV was examined on mitotic HT1080 cells with metaphase chromosomes. Extensive αVβ3 expression and bound DNP-KSHV were detected on similar cell surface domains (Fig. 3C, D; arrows). The Manders colocalization coefficients M1 (KSHV/αVβ3) and M2 (αVβ3/KSHV) were 0.75 and 0.84, respectively. An optical section of the merged images in Figures 3C and D revealed obvious metaphase chromosomes and strong colocalization of αVβ3 and DNP-KSHV (yellow), particularly on microvilli at the cell surface (Fig. 3E, arrows).
Figure 3. Increased αVβ3 integrin and enhanced KSHV-binding to mitotic cells.
HT1080 cells were fixed and incubated with anti-β3 integrin antibody alone (A, B) or mixed with DNP-KSHV (C-E). The fluorescent signals were enhanced with TSA 488 alone (anti-β3, green) (A, B) or sequentially with TSA 488 (anti-β3, green) and TSA 594 (anti-DNP, red)(C-E). Nuclear DNA was stained with TO-PRO 3 (blue). Mitotic cells in metaphase were identified based on condensed chromatin structure, while interphase (G0/G1) cells contained diffuse chromatin and intact nuclear membranes. A) αVβ3 expression (green) on a mitotic cell (arrowhead) compared to surrounding interphase cells. Inset shows condensed chromatin of the mitotic cell, blue channel only. B) quantitation of αVβ3 pixels/cell for interphase (n=413) and metaphase (n=42) cells, shown as the median value for the different cell types in each of 17 micrograph fields. C, D) Localization of αVβ3 (C, green) and bound DNP-KSHV (D, red) on a mitotic cell; maximum projection of confocal images (Z=20μm) with arrows marking similar structures. E) Colocalized pixels of images in C and D (yellow); section of merged confocal image (Z=0.5μm) to illustrate metaphase chromosomes (blue) and colocalization on microvilli (arrows). Bar = 10μm
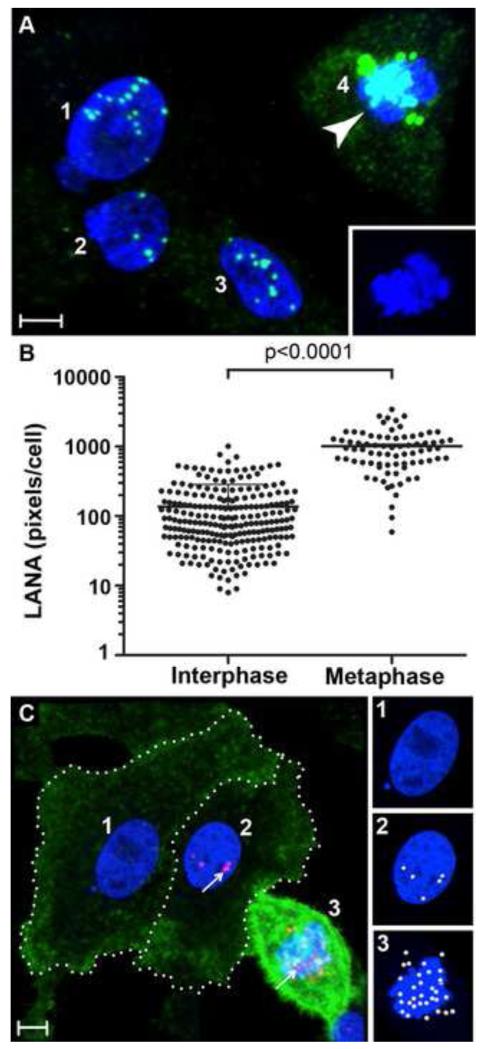
To determine whether there was a correlation between αVβ3 expression on mitotic cells and susceptibility to infection, HT1080 cells were incubated with unlabeled infectious KSHV for three hours. Twenty-four hours post-infection, the cells were fixed and stained for nuclear KSHV LANA (green), a marker of latent infection and TO-PRO 3 (blue) to visualize the nuclear DNA. Figure 4A shows a confocal micrograph of four adjacent numbered cells. Cells #1-3 had diffuse chromatin (blue) with intact nuclear membranes characteristic of interphase cells and contained small punctate LANA dots (green). Cell #4 had condensed chromatin and no nuclear membrane, characteristic of a mitotic cell (see inset Fig. 4A) with large, highly fluorescent LANA dots (green). To compare the LANA expression levels, 235 interphase and 77 metaphase cells were identified and the LANA fluorescent pixels were quantitated. The interphase cells had a mean of 136 LANA pixels/cell, while the metaphase cells had a mean of 1011 LANA pixels/cell (Fig. 4B). The large increase in LANA expression by metaphase cells was statistically significant (P<0.0001).
Figure 4. Increased KSHV LANA expression in infected mitotic cells with up-regulated αVβ3 integrin.
A-C) HT1080 cells were infected with unlabeled KSHV and incubated for 24 hours. A, B) infected cells were fixed and stained for KSHV LANA (green) using TSA 488. Nuclear DNA was stained with TO-PRO 3 (blue). A) Representative LANA staining in mitotic cell #4, (arrowhead) and interphase cells #1-3. Inset shows condensed chromatin of the mitotic cell #4, blue channel only. B) Quantitation of LANA pixels/cell for interphase (n=235) and metaphase (n=77) cells showing mean and standard deviation. C) infected cells were sequentially stained for cell surface αVβ3 integrin (green) and nuclear KSHV LANA (red) using TSA 488 and TSA 594, respectively. Cells #1 and 2 (dotted outlines) had nuclei with diffuse chromatin characteristic of interphase cells, while cell #3 had condensed chromatin characteristic of a mitotic cell. The individual nuclei of cells #1-3 are shown at the right (insets) with LANA dots rendered as spheres with the green fluorescence channel off to better visualize the chromatin. Quantitation of LANA dots revealed 0, 5 and 32 LANA dots in cells #1, 2 and 3, respectively. This data is representative of the staining on other cells in the culture. Bar = 10μm
To correlate LANA expression with the presence of cell surface αVβ3 on mitotic cells, KSHV-infected HT1080 cells were sequentially stained 24 hours post-infection for nuclear LANA and αVβ3, using the anti-LANA and anti-β3 monoclonal antibodies, respectively, with TSA enhancement. Metaphase and interphase cells were identified by TO PRO-3 staining of nuclear DNA. Integrin αVβ3 (green) was highly expressed on KSHV infected mitotic cells with metaphase chromosomes (blue)(Fig. 4C, cell #3) compared to interphase cells (Fig. 4C, cells #1 and 2). These results are representative of the other infected cells in the culture. In this micrograph, the laser detector gain was decreased to reduce the over saturated fluorescence of cell #3, thereby decreasing the overall αVβ3 fluorescence detected in cells #1 and #2. Nuclear-associated LANA, evidence of KSHV infection, was detected in cells #2 and 3, but not cell #1 (Fig. 4C). Since the αVβ3 green fluorescence obscured the LANA fluorescence in cell #3, punctate LANA was rendered as spheres for the individual nuclei with the green fluorescence signal eliminated (Fig. 4C, insets). The LANA sphere counts for cells #1, 2, 3, were 0, 5, and 32, respectively, demonstrating a large increase in LANA dots in the mitotic cell #3 compared to the interphase cells #1 and 2. Furthermore, quantitation of the fluorescent LANA pixels in these cells showed that the mitotic cell #3 had significantly more LANA fluorescent pixels (2641) than the infected interphase cell #2 (627 pixels). Since LANA dots and fluorescence are reflective of the number of KSHV genomes in an infected cell [34], these results show that there is a higher level of KSHV infection in mitotic cells, which correlates with increased αVβ3 expression.
The fibrillar distribution of cell bound KSHV correlates with actin-associated αVβ3 integrin in lamellipodia, filopodia, and focal adhesions
The cytoplasmic tail of β3 integrin acts as an attachment scaffold for a large number of molecules including talin, vinculin, α-actinin, filamin and tensin [35]. These molecules link αVβ3 integrin to the actin cytoskeleton. We recently showed that KSHV binds to actin associated cell surface microdomains, including filopodia, lamellipodia, ruffled membranes, microvilli and intercellular junctions [32]. Therefore, we compared the localization of αVβ3 and cell bound DNP-KSHV on structures known to contain actin to determine if they were both distributed in a fibrillar pattern. HT1080 cells were incubated with DNP-KSHV and stained for αVβ3, as described above. The cell margin of an HT1080 cell is shown in the top panels of Figure 5 (A-C). A “fibrillar” staining pattern for αVβ3 integrin (green) was detected at the cell margin (Fig. 5A, arrows). Cell surface bound KSHV (red) had a very similar pattern (Fig. 5B, arrows), suggesting that KSHV could be linked to actin through αVβ3 integrin. The colocalization coefficients M1 (KSHV/αVβ3) and M2 (αVβ3/KSHV) were 0.995 and 0.817, respectively. A surface rendering of the colocalized αVβ3 and KSHV pixels showed a distinct fibrillar pattern. (Fig. 5C, yellow). The finger-like actin rich filopodia protrusions extending from the cell margin and contacting the substratum are shown in the middle panels of Figure 5 (D-F). These structures were highly enriched for αVβ3 integrin, which was often organized in small nodes or “beads on a string” (Fig. 5D; arrow). KSHV also bound to filopodia (Fig. 5E), and the punctate pattern of bound KSHV largely matched the αVβ3 integrin pattern (Fig. 5F, yellow).
Figure 5. KSHV colocalizes with αVβ3 integrin on fibrillar cell structures.
Fixed (A-F) or viable (G-I) HT1080 cells were incubated with anti-β3 antibody and DNP-KSHV, and the fluorescent signals were sequentially enhanced with TSA 488 (anti-β3, green) and TSA 594 (anti-DNP, red). Virus/receptor localization was examined by confocal microscopy of the cell margin (A-C: low magnification; D-F: high magnification) and cell adhesion sites (G-I). A, D, G: αVβ3 (green); B, E, H: DNP-KSHV (red); C, F, I: colocalization (yellow), where C shows the surface rendering of colocalized fibrillar αVβ3 and KSHV pixels. A-C: lamellipodia (open arrows) with distinct fibrillar structures (arrows); D-F: small nodes of αVβ3 integrin and KSHV along filopodia (arrows). G-I) Cell footprint after HT1080 detachment following DNP-KSHV and anti-αVβ3 binding. Focal adhesions exposed at sites of lamellipodia retraction (arrows) are indicated, and the area under the cell body (star, white outline) which was accessible to the β3 antibodies but not the DNP-KSHV is shown. Inset shows a zoomed image of the adhesions with colocalized αVβ3 and KSHV (yellow). Bar=10μm
In live cell assays, incubating HT1080 cells with anti-β3 and KSHV at 4°C often caused lamellipodia retraction, destabilization of cell adhesion, and ultimately cell detachment and loss during fixation. The cell footprint remained on the dish (Fig. 5G-I, indicated with an “*”) with an outline marking the edge of the cell body before detachment. The focal adhesions from the cell footprint that were exposed at sites of lamellipodia retraction were highly enriched with αVβ3 integrin (Fig. 5G, arrow) and also bound DNP-KSHV (Fig. 5H; arrow). anti-β3 also identified focal adhesions under the cell body (Fig. 5G, “*” region), which were inaccessible to DNP-KSHV due to the size of the virion (150 nm), which prevented trafficking under the cell body during the virus binding step (Fig. 5H). A high degree of colocalization of αVβ3 integrin and KSHV was detected in focal adhesions within the lamellipodia retraction zone, but not under the cell body (Fig. 5I, yellow). Zoomed image of the lower left area is shown in the inset.
In addition to αVβ3, KSHV-binding sites are enriched for CD98, αVβ5 and α3β1 integrins, and the lipid raft marker, cholera toxin B, but not caveolin-1
To determine whether the cell surface microdomains binding KSHV contained other putative KSHV receptors in addition to αVβ3, we examined the colocalization of DNP-KSHV with cell surface CD98 and integrins α3β1 and αVβ5. HT1080 cells were incubated with DNP-KSHV and mouse antibodies to either CD98, α3β1 or αVβ5, and the fluorescent signals were sequentially enhanced with TSA 647 (anti-DNP) and TSA 488 (anti-cell surface markers), and colocalized pixels were pseudo-colored white. For these live cell assays, viable HT1080 cells were brought to 4°C and incubated with a mixture of DNP-KSHV and one of the individual primary antibodies, as described in Materials and Methods. The cultures were washed, fixed and the primary antibodies were detected using anti-mouse IgG-HRP and TSA 488. Cell bound DNP-KSHV was then localized using rat anti-DNP, goat anti-rat IgG-HRP and TSA 647, as described above.
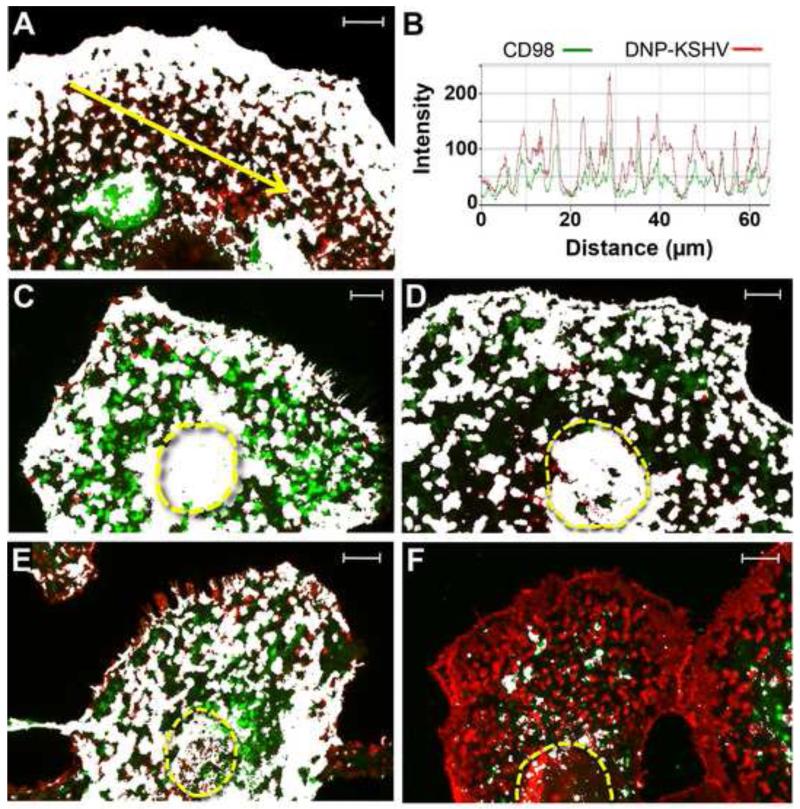
Confocal analysis revealed that the distribution of CD98 (green) overlapped extensively with bound KSHV (red) (Fig. 6A, colocalization – white). Manders colocalization coefficients were determined: M1 = 0.70 and M2 = 0.90, where M1 indicates the fraction of DNP-KSHV detected that overlaps with CD98 (KSHV/CD98), and M2 indicates the fraction of CD98 detected that overlaps with DNP-KSHV (CD98/KSHV). Thus, ~70% of KSHV bound to sites containing CD98, while 90% of the sites containing CD98 bound KSHV. A histogram of individual fluorescent intensities along the arrow in 6A shows that the DNP-KSHV peaks (red) identifying dorsal surface microdomains overlap the CD98 peaks (green) (Fig. 6B). The distribution of α3β1 integrin (green) also overlapped extensively with the KSHV-binding sites (red), particularly in the supranuclear domain (Fig. 6C, colocalization – white). Manders colocalization coefficients were determined: M1 = 0.955 and M2 = 0.726, where M1 indicates the fraction of DNP-KSHV detected that overlaps with α3β1 (KSHV/α3β1) and M2 indicates the fraction of α3β1 that overlaps with KSHV (α3β1/KSHV). Thus, more than 95% of the KSHV-binding sites contained α3β1. Integrin αVβ5 (green), another RGD binding integrin, colocalized with DNP-KSHV (red) in supranuclear and dorsal cell surface microdomains (Fig. 6D, colocalization – white, M1 (KSHV/αVβ5) and M2 (αVβ5/KSHV) colocalization coefficients = 0.944 and 0.68, respectively).
Figure 6. KSHV colocalizes on cell surface domains with CD98, αVβ5 and α3β1 integrins, and the lipid raft marker cholera toxin B.
HT1080 cells were incubated with DNP-KSHV (red) and antibodies to either A) CD98, C) α3β1, D) αVβ5, (E) biotinylated cholera toxin B (bio-CTB), a marker of lipid rafts [36] or F) caveolin-1, a marker of caveolae. The fluorescent signals were sequentially enhanced with TSA 488 (antibodies to cellular proteins and cholera toxin B, green) and TSA 647 (anti-DNP, red), and the merged images are shown (colocalized pixels, white). Supranuclear domains are indicated (yellow dashed outline). Manders colocalization coefficients M1 and M2 were A) CD98: 0.70/0.90; C) α3β1: 0.955/0.726; D) αVβ5: 0.948/0.68; E) bio-CTB: 0.82/0.716 and F) caveolin-1: 0.039/0.42, where M1 indicates the percentage of DNP-KSHV detected that colocalizes with the cellular marker, and M2 indicates the percentage of the cellular marker detected that colocalizes with DNP-KSHV. Fluorescent intensities for CD98 and KSHV along the arrow in panel A are shown in B. Bar=10μm
To further examine the constituents of the KSHV-binding microdomains, HT1080 cells were incubated with DNP-KSHV and either biotin labeled cholera toxin B (bio-CTB), a marker of lipid rafts [36], or antibodies to caveolin-1, a component of caveolae [37] and fluorescence was detected as above. DNP-KSHV colocalized with bio-CTB on the dorsal surface microdomains (Fig. 6E, colocalization – white). Manders colocalization coefficients of M1 = 0.82 and M2 = 0.71 were determined, where M1 is the fraction of DNP-KSHV detected that overlaps with the lipid raft marker (KSHV/CTB) and M2 is the fraction of the lipid raft marker that overlaps with DNP-KSHV (CTB/KSHV). These findings suggest that the KSHV-binding domains on the dorsal surface may represent lipid raft aggregates. Analysis of the localization of caveolin-1 and DNP-KSHV yielded Manders colocalization coefficients of M1 = 0.039 and M2 = 0.42, where M1 is the fraction of DNP-KSHV fluorescence detected that overlaps with caveolin-1 (KSHV/caveolin) and M2 is the fraction of caveolin-1 fluorescence detected that overlaps with DNP-KSHV (caveolin/KSHV)(Fig. 6F, colocalization white). These results demonstrated that the vast majority of the microdomains binding KSHV did not contain caveolin-1, suggesting that these KSHV-binding domains were not associated with caveolae of the endocytic pathway during the initial KSHV attachment. However, approximately 40% of the detected caveolin-1 was present in KSHV-binding domains.
KSHV binds to cell surfaces independent of heparan sulfate (HS)
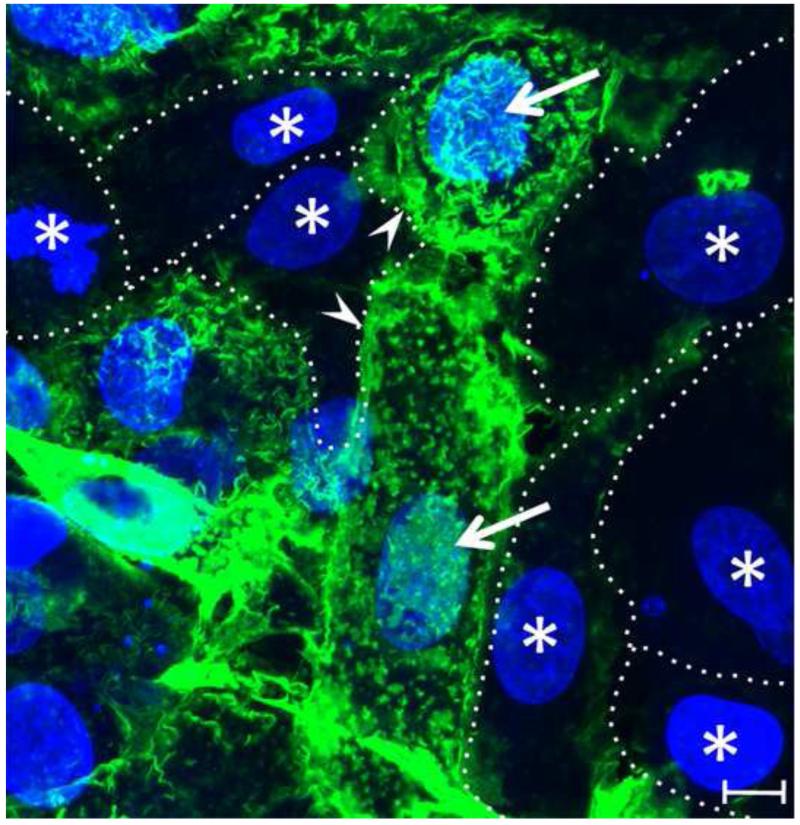
Since cell attachment of many herpesviruses, including KSHV, is thought to be initiated by the binding of virion envelope glycoproteins to cell surface heparan sulfate (HS), we examined the distribution of HS on HT1080 cells using monoclonal antibody 10E4, which is specific for N-sulfated residues in HS [38]. Confocal immunofluorescence microscopy revealed that the HT1080 cell population was highly heterogeneous in HS expression. Some cells displayed strong HS fluorescence particularly over the nucleus (Fig. 7, arrow) and at intercellular junctions (Fig. 7, arrowhead), similar to the domains binding KSHV in our earlier study [32]. However, adjacent cells (Fig. 7, indicated with “*”) showed much less staining with essentially no HS detected on the dorsal cell surface, intercellular junctions or in the supranuclear region.
Figure 7. Localization of cell surface heparan sulfate.
HT1080 cells were fixed and incubated with anti-HS antibody (10E4) followed by TSA 488 (green) enhancement to detect cell surface HS. Supranuclear membrane areas (arrow) and intercellular junctions (arrowheads) are indicated. Cells with essentially undetectable levels of HS are labeled with “*” and outlined. Bar = 10μm
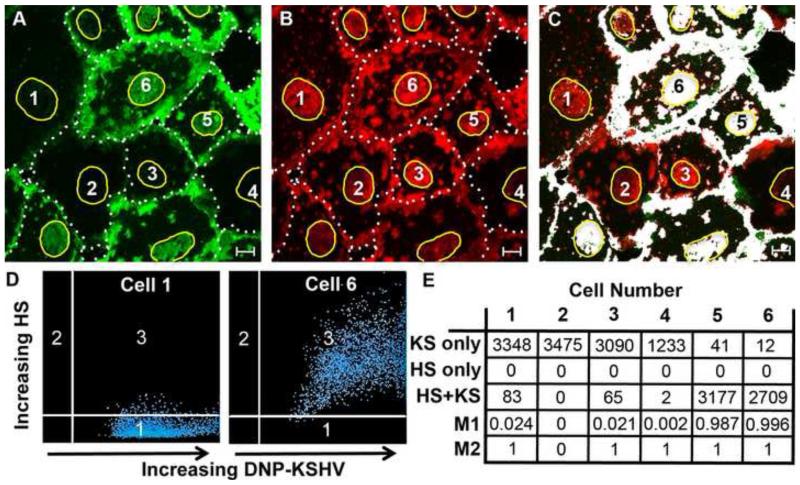
To determine if KSHV binds preferentially to the HS expressing HT1080 cells, fixed HT1080 cells were incubated with DNP-KSHV and sequentially stained with antibody 10E4 and TSA 488 (green) followed by anti-DNP and TSA 647 (red), as described in Materials and Methods. Figure 8 shows separate micrographs of the distribution of HS (Fig. 8A, green), KSHV (Fig. 8B, red), and colocalization (Fig. 8C, white) of the same field. The cell borders (white dashed line) and the outline of ethidium stained nuclei (solid yellow line) are indicated. Several cells, representative of the whole cell culture, have been numbered 1-6 to illustrate the heterogeneity of HS expression. Abundant junctional and supranuclear HS staining (green) were detected in cells #5 and 6, as well as staining of dorsal cell surface microdomains. In contrast, cells #1-4 showed extremely low levels of HS with essentially no supranuclear or dorsal microdomain staining. The distribution of HS and bound KSHV on cells #5 and 6 was remarkably similar, as shown by the extensive colocalization in Figure 8C (white pixels). Interestingly, cells #1-4 with low HS showed high levels of bound KSHV similar to that seen in cells #5 and 6 (Fig. 8B), which had high levels of HS. When HS was present on the cell surface, KSHV colocalized with HS on the same microdomains (Fig. 8C). The low level of green HS pixels in Figure 8C indicated that a high proportion of the HS sites had bound KSHV. It is noteworthy that KSHV was still found at supranuclear, intercellular junctions, and dorsal membrane regions whether HS was present (cells #5 6) or not (cells #1-4).
Figure 8. Localization of HS and KSHV-binding sites on cell surface structures.
Fixed HT1080 cells were incubated with DNP-KSHV and stained with anti-HS antibody (10E4) using sequential enhancement with TSA 647 and TSA 488, respectively. The micrographs are of the same field showing the distribution of A) HS (green), B) DNP-KSHV (red), and C) colocalization (white). Cell borders are indicated with a dashed line. D) Scattergrams comparing pixel intensities of cells #1 and 6; KSHV (quadrant 1), HS (quadrant 2), and colocalized pixels (quadrant 3). The yellow outline in A-C marks the nuclear edge within which a 65μm2 ROI for cells #1-6 was selected for the quantitation. E) pixel counts in the R0I for KSHV-only (red), HS only (green), HS+KSHV colocalized pixels (white). Manders colocalization coefficients (M1 and M2) were determined for each cell within the ROI, where M1 indicates the percentage of bound KSHV that overlaps with HS and M2 indicates the percentage of HS-containing sites that overlap with bound KSHV. Bar = 10μm
To quantify the HS and KSHV colocalization and derive colocalization coefficients, fluorescent pixels (red and green) were analyzed within a 65 μm2 supranuclear membrane area (ROI) over the ethidium-stained nuclei of cells #1-6. This region was selected to ensure that the membrane area to be analyzed was from a similar morphologic region and that there was no chance of membrane overlap with an adjacent cell. Figure 8D shows the ROI scattergrams for cells #1 and 6 where the x-axis shows increasing KSHV pixel intensity (quadrant 1), the y-axis shows increasing HS pixel intensity (quadrant 2), and the colocalized pixels are present in quadrant 3. The pixels in cell #1 partitioned almost exclusively to the KSHV quadrant 1, while the pixels in cell #6 partitioned to the KSHV/HS colocalization quadrant 3. A table of the pixels counts for KSHV-only (red), HS only (green), and colocalized KSHV/HS (white) pixels, and Manders colocalization coefficients is shown in Figure 8E, where M1 indicates the fraction of DNP-KSHV detected that overlaps with HS (KSHV/HS), and M2 indicates the fraction of HS that overlaps with KSHV (HS/KSHV). The total pixel count for cells #1-3, 5 and 6 was remarkably similar. Only a partial view of cell #4 was visible in the micrograph therefore the total pixel count was less. Cells #1-3, which expressed low levels of HS, bound equivalent levels of KSHV as cells #5 and 6, which expressed high levels of HS (KSHV pixels: cell #1, 3431; cell #2, 3475; cell #3, 3155; cell #5, 3218; cell #6, 2721). When HS was present (cells #5 and 6), the distribution of bound KSHV highly correlated with the localization of HS (M1 colocalization coefficients (KSHV/HS) = 0.987 and 0.996 for cells 5 and 6, respectively). In contrast, in cells #1-4 the distribution of bound KSHV showed little correlation with the localization of HS (M1 colocalization coefficients (HS/KSHV) = 0.024, 0, 0.021. and 0.002 for cells #1-4), indicating that virus binding was not dependent on HS.
Human salivary gland (HSG) epithelial cells are highly resistant to KSHV infection and lack KSHV-binding domains
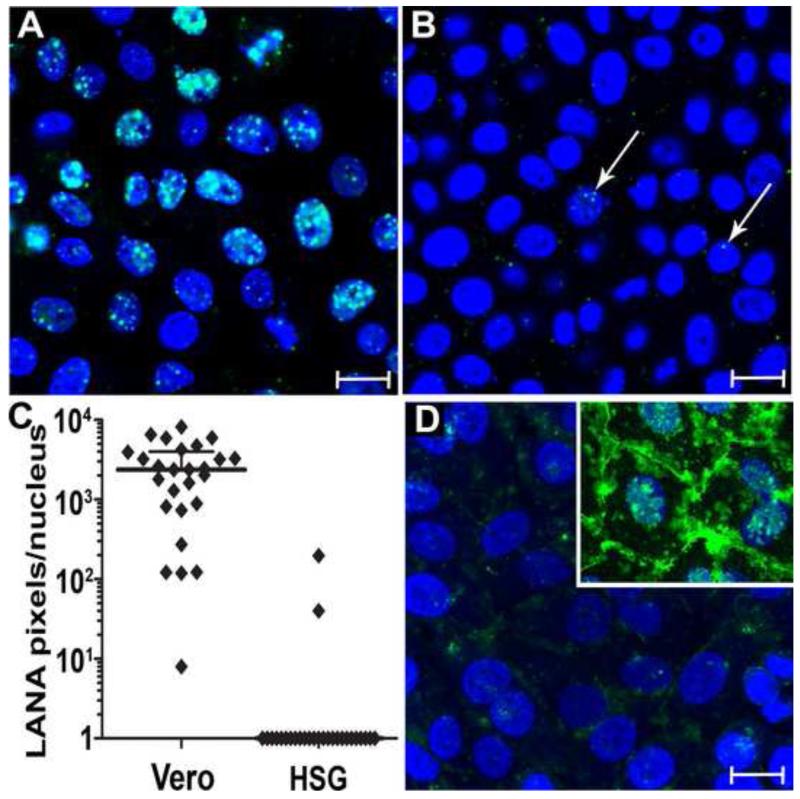
The strong correlation between membrane associated αVβ3 integrin and cell structures binding KSHV supports our previous data that αVβ3 integrin binds directly to purified KSHV virions through an RGD mediated interaction with glycoprotein B [30]. To further examine the role of αVβ3 in the initial binding and entry of KSHV, we screened a number of adherent cell lines for resistance to KSHV infection, using KSHV LANA as a marker of infection, as described previously [30]. The human salivary gland (HSG) epithelial cell line [39] was identified as highly resistant to KSHV infection. To compare HSG cells to Vero cells, which we and others have shown to be highly susceptible to KSHV infection, identical aliquots of gradient purified unlabeled KSHV were incubated with confluent cultures of either Vero or HSG cells. The majority of the Vero cells (95%) were infected with KSHV with many expressing high levels of punctate nuclear LANA with strong fluorescence (Fig. 9A). Under the same conditions, 97% of the HSG cells showed no detectable LANA fluorescence. In those few cells with apparent LANA fluorescence, only minimal numbers of LANA nuclear dots were observed, which were very weakly fluorescent (Fig. 9B). Quantitation of the LANA nuclear pixels revealed very high levels in Vero cells (median = 2367 pixels) compared to HSG cells (median = 0 pixels)(P<0.0001; Mann-Whitney U test)(Fig. 9C). Since previous studies have shown that the number and summed immunofluorescence of LANA dots in the cell nuclei are directly proportional to the amount of intracellular KSHV DNA in the infected cell [34], our data strongly demonstrate that HSG cells are highly resistant to KSHV infection.
Figure 9. HSG epithelial cells are resistant to KSHV infection and lack KSHV-binding domains.
A) Vero or B) human salivary gland (HSG) epithelial cells were incubated with gradient purified KSHV (~1,000 genomes/cell) for 3 hours, washed, and incubated for 24 hours. The cells were fixed and reacted with an antibody to KSHV LANA to detect latent infection, and fluorescence was enhanced using TSA 488 (green). Nuclei were stained with TO PRO-3 (blue). C) LANA expression was quantitated by counting fluorescent pixels per nucleus. Vero cells (median = 2367 pixels/nucleus); HSG cells (median = 0.0 pixels/nucleus) (P<0.0001; Mann-Whitney U test). D) DNP-KSHV was incubated with HSG cells or control HT1080 cells (inset) and bound virus was detected using anti-DNP and TSA 488 (green). Bar = 20 μm
To determine whether HSG cells were impaired in their ability to bind KSHV during the initial step of virion attachment, confluent HSG and HT1080 cell cultures were fixed and incubated with DNP-KSHV, as described in Figures 1-3. Whereas DNP-KSHV bound strongly to HT1080 cells (Fig. 9D, insert), as described above, little or no binding was detected on HSG cells (Fig. 9D).
HSG cells express all of the putative KSHV receptors except αVβ3 integrin
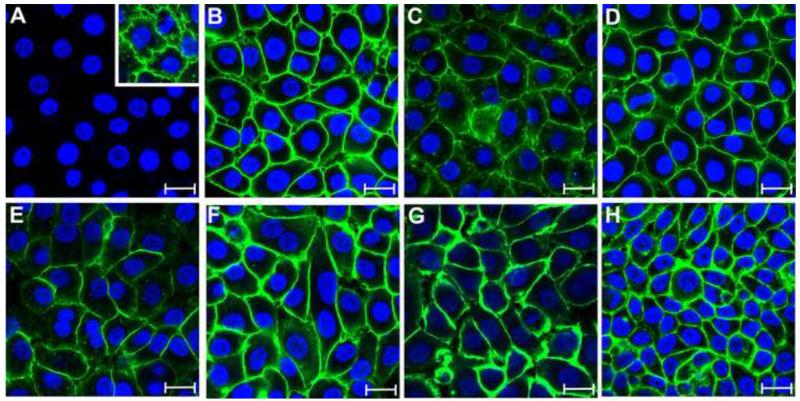
To further characterize the HSG cells, we screened for the presence of αVβ3 integrin and other cell surface proteins proposed as receptors for KSHV using confocal microscopy with antibodies specific to the different receptor proteins. Whereas αVβ3 integrin was easily detected on Vero cells using the LM609 monoclonal antibody that recognizes the αVβ3 integrin heterodimer (Fig. 10A, inset), essentially no reactivity was detected on HSG cells (Fig. 10A). In contrast to αVβ3, HSG cells strongly expressed α3β1 integrin (Fig. 10B), cystine transporter xCT light chain (Fig. 10C) and CD98 heavy chain (Fig. 10D), HS (Fig. 10E), the HS-proteoglycan, syndecan (Fig. 10F), αVβ5 integrin (Fig. 10G) and the EphA2 receptor (Fig. 10H). Thus, the resistance to KSHV infection and the lack of KSHV-binding sites on HSG cells correlated with a lack of cell surface αVβ3 integrin.
Figure 10. Human salivary gland (HSG) epithelial cells express high levels of the putative KSHV receptors, except αVβ3 integrin.
HSG cells were incubated with antibodies reactive with the different cell surface proteins proposed as entry receptors for KSHV, including A) αVβ3 integrin (LM609), B) α3β1 integrin, C) cystine transporter light chain xCT, D) heavy chain CD98, E) HS, F) HS proteoglycan syndecan, G) αVβ5 integrin, and H) EphA2. The expression of the putative lymphocyte receptor, DC-SIGN, was not determined. Inset in panel A: Vero cells incubated with antibody to αVβ3 integrin (positive control). The fluorescent signals were enhanced using TSA 488. Nuclei TO-PRO 3 (blue). The same laser power setting was used throughout. The images are confocal sections to allow comparisons of fluorescent intensities, and are representative of the intensity of staining of the dorsal cell surfaces in maximum projection images. Bar = 20μm
Ectopic expression of αVβ3 integrin renders HSG cells susceptible to KSHV infection
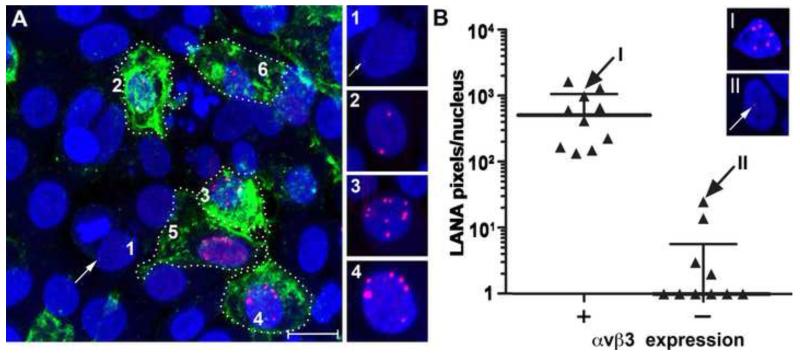
To examine the role of αVβ3 integrin in KSHV entry, HSG cells were transfected with a pCDNA3 construct expressing β3 integrin to reconstitute cell surface αVβ3 integrin expression. Twenty four hours post transfection, the cells were infected with gradient purified unlabeled KSHV. After 24 hours, the cells were stained for cell surface αVβ3 integrin and then fixed and stained for nuclear KSHV LANA. A representative micrograph (Fig. 11) shows a number of transfected cells that express cell surface αVβ3 (green, outlined) along with a number of non transfected cells that only stained with nuclear TO-PRO 3 (blue). Abundant KSHV LANA protein was detected in cells that expressed αVβ3 integrin (Fig. 11A, e.g. cells #2-6). These cells contained nuclear LANA dots with high levels of LANA (red) fluorescence. In contrast, none of the αVβ3-negative HSG cells had significant LANA expression. Only a few cells showed a possible single LANA dot, which was barely visible in the micrograph (see Fig. 11A, cell #1-arrow). Micrographs of the individual nuclei (#1-4) are shown without the αVβ3 signal to demonstrate the difference in the level of KSHV LANA protein.
Figure 11. Ectopic expression of αVβ3 integrin renders HSG cells susceptible to KSHV infection.
HSG cells were transfected with a β3 integrin expression plasmid and subsequently infected with KSHV, as described in Methods. A) Cell surface β3 integrin was detected on live cells using mAB B3A (green). The cells were then fixed and nuclear KSHV LANA was detected using mAB LN53 (red). Fluorescent signals were sequentially enhanced using TSA 488 and TSA 594, respectively. Nuclei were counterstained with TO-PRO 3 (blue). The cell borders of a number of transfected HSG cells expressing αVβ3 are marked with a dotted line. One untransfected (αVβ3-negative) cell (#1) and five transfected (αVβ3-positive) cells are numbered (2-6). Fluorescent staining of the nuclear LANA protein is shown in the montage of individual nuclei at the right with the 488 (green) laser off. A very weak LANA fluorescent spot was detected in the untransfected cell #1 (arrow). B) LANA expression was quantitated by counting fluorescent pixels per nucleus for αVβ3 expressing and non-expressing HSG cells. αVβ3-positive cells (median = 510 pixels/nucleus); αVβ3-negative cells (median = 0 pixels/nucleus) (P=0.0001; Mann-Whitney U test). Micrographs of the cell nuclei corresponding to data points “I” (αVβ3-positive; 991 LANA pixels) and “II” (αVβ3-negative; 25 LANA pixels) are shown for comparison. Bar = 20 μM.
LANA pixels were quantitated for the αVβ3-positive and αVβ3-negative HSG cells. Nuclear LANA levels in individual αVβ3-expressing cells (n=11) varied from 134 to 1640 pixels/nucleus with a median of 510 pixels (Fig. 11B). In contrast, most of the αVβ3-negative cells (6/10) had no detectable LANA fluorescence. Quantitation of nuclear areas within the αVβ3-negative cells showed minimal nuclear LANA pixels (median = 0.0 LANA pixels/nucleus), which was significantly less than the 510 median nuclear LANA pixels seen in the αVβ3 integrin-positive cells (P=0.0001). Micrographs of individual nuclei and their corresponding data points are given to illustrate the difference in LANA detected in αVβ3-positive cells (Fig. 11B, “cell I”) and αVβ3-negative cells (Fig. 11B, “cell II”). Overall, ectopic expression of αVβ3 integrin rendered the HSG cells highly susceptible to robust infection by KSHV with high numbers of LANA dots and high levels of LANA fluorescence.
DISCUSSION
Previously we showed that KSHV binds to actin-based cell surface structures on cells in interphase, including filopodia, lamellipodia, and ruffled membranes at the cell margin, as well as dorsal surface “island” domains and a specific supranuclear region [32]. KSHV-binding was up-regulated on mitotic cells and the virus associated with extensive microvilli over the entire cell surface indicating that the binding domains are highly mobile structures that change in density and distribution during the cell cycle. In the present study, we have investigated the receptors mediating the initial KSHV cell binding.
Historically, HS has been implicated as an attachment factor for KSHV based on studies showing that soluble heparin blocked virus infection [17,31] and that cells treated with heparinase to remove cell surface HS showed decreased binding of KSHV virions [40] and KSHV infection [31]. Analyzing individual HT1080 cells by confocal microscopy, we found that KSHV-binding was not dependent on HS, as virus bound equally to cells with and without HS that were present in the same cell culture. Not only was the amount of KSHV-binding similar between cells with and without HS, but the virus localized to similar cellular membrane domains regardless of the presence of HS. The lack of correlation of KSHV-binding and HS expression was further confirmed in our studies of the HSG cells, which expressed high levels of HS and the HS containing proteoglycan syndecan, yet bound extremely low levels of KSHV and were resistant to KSHV infection following a 3hr incubation with high titer virus. Interestingly, the KSHV glycoprotein H/glycoprotein L complex, which has been implicated in virus entry, was found to adhere to heparan sulfate negative cells at lamellipodium-like structures [19].
We showed previously that the binding of KSHV to HT1080 cell cultures containing HS-positive and HS-negative cell surface domains was inhibited by heparin treatment [32]. While the ability of heparin and heparinase to inhibit infectivity of KSHV has been attributed to inhibition of virus binding to HS containing cell surface molecules, recent reports have shown that these treatments have a broad range of effects on cells. Heparin/heparan sulfate directly binds αVβ3, αVβ5, and α5β1 integrins with high affinity and blocks the binding site for RGD ligands [41]. Heparin also binds the integrin αxβ2 and induces conformational changes in the receptor ectodomain [42]. Heparin induced conformational changes of integrin receptor clustering, and cytoskeletal rearrangements have been observed in other systems [43]. Heparin can also induce cell signaling pathways leading to the phosphorylation of focal adhesion proteins [44]. The removal of HS moieties by heparinase treatment can also cause wide ranging cellular changes, including decreased stress fibers and focal adhesions leading to increased filopodia formation and cell migration [45] and blocked recruitment of α5β1 integrin into lipid rafts [46]. Thus, the ability of heparin and heparinase to block KSHV-binding and infection may be due to indirect effects on the virus binding domains or on the structure of the viral envelope itself in addition to specific interactions with HS receptors.
Previously, we identified the integrin αVβ3 as a candidate entry receptor for KSHV. We determined that affinity-purified αVβ3 receptor bound to intact KSHV virions recognizing an RGD motif within the envelope glycoprotein B, and KSHV infections were inhibited by soluble RGD peptides and anti αVβ3 antibodies (30). Our current study clearly showed that KSHV bound to membrane domains containing αVβ3. Approximately 90% of KSHV bound to cell surface domains colocalized with αVβ3 while 80% of cell surface αVβ3 colocalized with bound KSHV. KSHV-binding was enriched on actin-based fibrillar structures throughout the lamellipodium, and in many instances the virus was distributed in a “fibrillar” pattern colocalizing with αVβ3 integrin. KSHV also colocalized with integrin αVβ3 on microdomains on the dorsal cell surface as well as in supranuclear domains, while membrane areas with no detectable αVβ3 were essentially devoid of KSHV. The ratio of red (DNP-KSHV) and green (αVβ3) fluorescent signals within specific cell surface domains was sometimes variable across the cell, producing shades of yellow colocalization: red tinged in dorsal and supranuclear cell surface domains and green tinged in cell membrane edges and junctional regions. We believe that the variable ratio of red DNP-KSHV to green αVβ3 fluorescence in colocalization domains was due to small changes in the relative intensity of the two probes, which altered the color of the merged image and the perception of probe colocalization [47]. The color variation suggests that the two probes are not present in a fixed and equal proportion across the cell surface microdomains. We used multiple approaches to analyze colocalization including i) side by side comparisons of two images with arrow landmarks, ii) profile histograms of individual probe intensities along a defined region of the cell, iii) scatterplots of the intensities of both colors, and iv) Manders colocalization coefficients based on unbiased automatic thresholding. In all cases, the accurate measurements of DNP-KSHV and αVβ3 receptor fluorescence demonstrated strong colocalization within the specific cell surface binding domains.
We also detected a high level of colocalization of αVβ3 and KSHV-binding to cell surface structures on mitotic cells. Previously, we determined that KSHV-binding was increased on mitotic cells compared to cells in interphase [32]. In the current study, we detected increased expression of αVβ3 on mitotic HT1080 cells, confirming previous studies in rat fibroblasts [33]. We also found that KSHV-infected mitotic cells had higher levels of LANA than infected interphase cells. Since LANA levels have been shown to be proportional to the amount of intracellular viral DNA [34], our studies suggest that increased KSHV uptake by mitotic cells could be mediated by abundant cell surface αVβ3 integrin.
Our studies suggest that the association of KSHV with known actin-containing cell surface domains and fibrillar structures is due to interactions with αVβ3 integrin indirectly anchored to the cytoskeleton. These findings are consistent with previous studies describing the role of actin dynamics in regulating multiple steps in KSHV entry and intercellular trafficking [21,23]. The cell surface binding of KSHV induces integrin signaling through the focal adhesion kinase (FAK) present in actin associated focal adhesions, and FAK activation and downstream signaling has been implicated in actin rearrangement, endocytosis and KSHV entry [48,49]. Integrin signaling is believed to play a critical role in subsequent steps in the establishment of KSHV infection and new evidence suggests that αVβ3 is induced during latent KSHV infection and activates angiogenic phenotypes that are associated with KS tumor formation [50].
We screened cell lines for resistance to KSHV infection and analyzed cell surface receptor profiles to study the cause of the resistance. While most epithelial cell lines are susceptible to KSHV infection, we observed that the human salivary gland epithelial cell line (HSG) was highly resistant. In addition, we observed that HSG cells lacked the KSHV-binding sites on the cell surface that we detected using DNP-KSHV on other cell types. We determined that the lack of KSHV-binding sites correlated with a lack of cell surface integrin αVβ3 expression on the HSG cells. In contrast, HSG cells expressed abundant levels of all of the other putative KSHV receptor proteins currently identified on non lymphoid cells, including the integrins α3β1 and αVβ5, HS, syndecan, xCT, CD98 and the EphA2 receptor. We reconstituted surface αVβ3 expression in HSG cells by transfection of a plasmid expressing human β3 integrin and found that transfected HSG cells expressing cell surface αVβ3 were now highly susceptible to KSHV infection, while cells lacking αVβ3 expression were not. While HSG cells lacking αVβ3 expression are resistant to KSHV infection, they are susceptible to infection by rhesus rhadinovirus (RRV) [51], a macaque homolog of KSHV, indicating that the membrane structures responsible for virus entry are functional. Interestingly, RRV lacks an “RGD” motif in its virion glycoprotein B, does not interact with either αVβ3 or α3β1 integrin [30], and uses clathrin mediated endocytosis for viral entry [52]. Coupled with our data that αVβ3 integrins colocalize with KSHV-binding sites and our previous data that purified αVβ3 integrin binds KSHV virions through interactions with the RGD motif of KSHV glycoprotein B [30], the αVβ3 reconstitution data supports a critical role for αVβ3 integrin in KSHV-binding and entry, which is consistent with a dual role as a binding and entry receptor.
It is widely known that B-lymphocyte cell lines, such as BJAB, are also highly resistant to KSHV infection in vitro, even though B-cells are considered to be the main viral reservoir in vivo. BJAB and other B-cell lines lack heparan sulfate expression, which has been linked to their inability to be infected by KSHV [53]. However, reconstitution of HS expression on BJAB cells did not alter their ability to be infected by KSHV, even though it promoted some cell surface binding [53]. This study concluded that BJAB cells lacked a specific KSHV surface receptor that was critical for virus entry. Interestingly, αVβ3 integrin was essentially undetected on BJAB cells [27,54]. We are currently testing whether expression of recombinant αVβ3 integrin on BJAB cells could reconstitute the cell surface KSHV-binding domains that we have detected on non-lymphoid cells.
Our studies showed a high degree of colocalization of the putative KSHV receptors, including integrins αVβ3, αVβ5, and α3β1, and CD98, the integrin interacting heavy chain of the xCT cystine transporter heterodimer, within the distinct cell surface microdomains that bind KSHV. All four proteins have been previously implicated in KSHV-binding and entry, and subsequent activation of integrin pathways. CD98 is a lipid raft associated protein that is required for entry of vaccinia virus using the endocytotic pathway [55]. We also determined that KSHV-binding sites colocalized strongly with the lipid raft marker, cholera toxin B suggesting that the majority of KSHV-binding domains could correspond to lipid raft aggregates. While we detected strong KSHV-binding to cell surface domains lacking heparan sulfate, when heparan sulfate was present, it also colocalized to the KSHV-binding domains.
Previous studies of non-viral systems have shown that the putative KSHV receptor proteins αVβ3, αVβ5, α3β1, and CD98 localize to similar cell membrane domains and functionally interact [56-64]. EphA2, another proposed KSHV receptor, localizes in focal adhesions and has been implicated in alterations of actin dynamics and cytoskeleton reorganization by negatively regulating integrins [65]. Thus, αVβ5, α3β1, CD98, EphA2 and the HS-containing proteoglycans may play important roles in KSHV entry through their close association and interactions with αVβ3 on the cell surface microdomains that bind KSHV. The present study was done in non-lymphoid cells and the role of DC-SIGN, a putative KSHV receptor in lymphoid cells [24,25], was not examined.
The exact role of integrin α3β1 in KSHV entry is controversial. Previous studies have shown that α3β1 functionally interacts with CD98 and EphA2 in KSHV entry [66] and can be immunoprecipitated in a complex with KSHV glycoprotein B [29]. This latter study concluded that α3β1 is a KSHV entry receptor and implicated RGD interactions as the basis for the formation of an α3β1 and glycoprotein B complex. However, the vast literature on α3β1 has clearly shown that α3β1 does not interact with its ligand through an RGD-mediated interaction [67-72]. In the context of KSHV entry, we have shown that soluble purified α3β1 receptor does not bind glycoprotein B on intact KSHV virions, nor does it bind a KSHV glycoprotein B peptide containing the RGD motif [30]. These results contradict the belief that α3β1 functions as a KSHV receptor through RGD interactions with glycoprotein B, and suggest that the co immunoprecipitation of α3β1 and KSHV glycoprotein B, the basis for this conclusion [29], was not the result of a direct interaction between α3β1 and glycoprotein B, but was due to interactions with other proteins present in the whole cell lysate used for the immunoprecipitation experiment. The direct binding of purified α3β1 receptor and KSHV glycoprotein B was not analyzed in this study. In the current study, we determined that αVβ3 negative HSG cells are resistant to infection even though they express high levels of α3β1. Reconstitution of cell surface αVβ3 expression by transfection of β3 integrin rendered the HSG cells highly susceptible to KSHV infection. This contrasts directly with our previous studies on α3β1, which showed that α3β1-null cells are fully competent for infection by KSHV and that reconstitution of α3β1 by transfection with β3 integrin actually reduces KSHV infectivity rather than enhances it [30]. However, it is clear from our binding studies that α3β1 is highly associated with KSHV-binding domains containing αVβ3, and may play a secondary role with αVβ3 in KSHV entry.
Recently it has been proposed that KSHV binds and enters cells using multiple different receptors in a time dependent manner [27,73]. Upon KSHV infection, xCT/CD98 was co precipitated with integrins αVβ3, αVβ5, and α3β1 suggesting that virus entry induced the molecular association, which was not observed in uninfected endothelial cells. However, it was not determined whether virion proteins were present in the multi molecular complex nor whether the complex was induced by a single KSHV-receptor interaction or if the virus bound directly to multiple receptors in the complex.
In conclusion, we found that CD98 and integrins αVβ3, αVβ5, and α3β1 are all present within the majority of KSHV-binding domains on HT1080 cells and therefore would be positioned to participate in a virus-induced multi-receptor complex. Our studies with HSG cells, which express a complex repertoire of putative KSHV receptors including HS and HS-containing proteoglycans, integrin α3β1 and αVβ5, xCT/CD98 heterodimer, and EphA2 receptor, showed that susceptibility to KSHV infection was dependent on the presence of αVβ3 integrin. This suggests that the binding of KSHV to αVβ3 integrin within specific cell surface microdomains initiates the formation of a multi-molecular complex of integrins and other cell surface receptors such as α3β1 and xCT/CD98, resulting in integrin-induced signaling and entry of the KSHV virion. Additional interactions between the KSHV glycoprotein H/glycoprotein L complex and the EphA2 receptor may also occur in this molecular complex [28,66]. Our previous studies demonstrated that RGD-dependent interactions between αVβ3 and KSHV glycoprotein B are a critical step in KSHV entry [30].
MATERIALS AND METHODS
Cell lines
The human HT1080 fibrosarcoma cell line was a gift of Dr. W. Carter. African green monkey Vero cells were from the American Type Tissue Culture. The human salivary gland (HSG) cells were originally isolated from an irradiated submandibular gland and grow as an undifferentiated monolayer with cuboidal morphology [39]. HSG cells were a gift from Dr. K. Izutsu. HT1080, Vero, and HSG cells were maintained in DMEM containing 10% fetal bovine serum (FBS). BCBL-1 cells latently infected with KSHV [74], were cultured in RPMI complete medium with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1.0 mM HEPES, and 0.01% 2-mercaptoethanol at 37°C.
Immunological reagents
The following mouse monoclonal antibodies were purchased from Chemicon: anti-β3 integrin (clone P1B5), anti-αVβ3 integrin (clone LM609), anti-β3 integrin (clone B3A), and anti-αVβ5 (clone P1F6). LM609 recognizes the αVβ3 heterodimer, while B3A recognizes the β3 chain. The R1 rabbit anti-xCT (P218 232; immunizing peptide = n-TQNFKDAFSGRDSSIC-c) antiserum, was a kind gift from Dr. F. Jenkins. Other antibody reagents included: goat anti-mouse IgG-HRP, goat anti-rat IgG-HRP, donkey anti-goat IgG-HRP, and non-immune mouse IgG (Jackson Immunochemicals); rat anti-LANA (clone LN53) (Advanced Biotechnologies); non-immune goat serum, biotin-cholera toxin B, and goat anti-biotin-HRP, (Sigma); mouse anti-heparan sulfate (10E4; Seikagaku); mouse anti-CD98 (clone 44D7; Serotec); mouse anti-syndecan 1 (clone B-A38; Abcam); rabbit monoclonal anti-Ephrin A2 (clone D4A2; Cell Signaling); goat anti-mouse IgG-G20 gold conjugate (BBInternational); monoclonal rat anti-DNP (clone LO DNP-2; Invitrogen); mouse anti-caveolin (BD Biosciences Pharmingen. Other reagents included TSA 488, TSA 594, TSA 647, TO-PRO 3, and SlowFade (Invitrogen).
KSHV labeling and purification
KSHV virions from culture medium of TPA-treated BCBL cells were concentrated approximately 200-fold by centrifugation onto an Opti-Prep cushion, as described previously [30]. In some experiments, aliquots of concentrated KSHV were labeled with NHS-dinitrophenol (DNP) (10 μg/ml) and purified by centrifugation on a 20%, 25%, 30%, and 40% Opti-Prep step gradient. Fractions were collected from the top of the gradient using a BioComp gradient station (BioComp Instruments, Inc., New Brunswick, Canada) and the peak of infectious virus (DNP-KSHV) was used in subsequent virus binding experiments, as previously described [32]. The corresponding gradient fractions of DNP-labeled medium from a mock virus-infected culture were used as a labeling control (DNP-mock).
KSHV infection
Vero, HT1080 or HSG cells were infected with unlabeled KSHV (~1000 genomes/cell) for 3 h at 37°C, washed, and incubated for an additional 24 h. The cultures were then fixed with 4% paraformaldehyde (PF) in PHEM/sucrose (60mM PIPES, 25mM Hepes, 10mM EGTA, 2mM MgCl2, 4% sucrose) for 30 min at 37°C. In some experiments, cell surface αVβ3 integrin was localized on KSHV infected cells prior to fixing, as described below. Free aldehydes were quenched with 50mM NH4Cl and the cells were permeabilized with 0.5% NP-40/0.2% Tween 20. Endogenous peroxidase activity was inhibited with 3% H2O2. The cells were incubated with 10% normal goat serum (NGS) followed by 10% milk containing 1% NGS (blotto/NGS) to block non-specific binding. To detect KSHV infected cells, the anti-LANA monoclonal antibody LN53 (1:500) was incubated with cells for 2.5 h. The cells were washed and bound antibody was detected with goat anti-rat IgG-HRP (1:100) followed by a 10 minute TSA amplification (Invitrogen) with TSA 488 or TSA 594 in colocalization experiments. The nuclei were stained with TO-PRO 3. The cells were analyzed by confocal microscopy and the percent of cells expressing nuclear LANA was determined manually (100-200 cells counted/group). The amount of LANA fluorescence associated with interphase and mitotic infected HT1080 cells was quantitated using the Zeiss LSM software histogram function. A region of interest (ROI) in each cell was selected and the total number of LANA pixels within the ROI was determined using a fluorescent intensity range of 100-255. The mean pixel count +/− standard deviation was calculated and the unpaired t test (two-tailed) was used to compare interphase and metaphase cells.
In some experiments, recombinant αVβ3 integrin was expressed in HSG cells prior to KSHV infection. The HSG cells were transfected with pcDNA3-β3 expressing human β3 integrin (clone #1492, kindly provided by Dr. M.H. Ginsberg; 100 ng per well) using Lipofectamine 2000. After 5 h, the transfection reagent was removed and the cells were cultured for 24 h. The cells were then infected with unlabeled purified KSHV, as above. Twenty-four hours post-infection, cell surface αVβ3 integrin was detected using a live cell assay, as described below in the receptor localization methods. The cells were permeabilized and nuclear KSHV LANA was detected and quantitated, as above using TSA 594.
Localization of cell-bound KSHV and cell surface receptors
Receptor localization
The expression of different putative KSHV receptors was examined on the surface of live HSG and HT1080 cells at 4°C, using antibodies to αVβ3 (LM609) (1:100), β3 (1:100), xCT (1:200), CD98 (1:100), HS (1:50), syndecan-1 (1:10), αVβ5 (1:50) and EphA2 (1:100). The cells were incubated with the anti-receptor antibodies for 1 h at 4°C and then fixed in 4% paraformaldehyde in PHEM/sucrose. Free aldehydes were quenched and endogenous peroxidase activity and non-specific binding was inhibited, as described above. The anti-receptor antibodies were detected with either goat anti-mouse IgG HRP or anti rabbit IgG-HRP and TSA 488 (10 min amplification). The rabbit monoclonal anti-EphA2 (1:100) recognized denatured receptor and therefore was used on fixed HSG cells.
Colocalization of receptors and bound KSHV
KSHV and cell surface receptor colocalization was done using either fixed or live cell assays. For colocalization of cell surface αVβ3 and DNP-KSHV in fixed cell assays, HT1080 cells were fixed, as described above, and incubated for 3 h with a mixture of mouse anti-β3 antibody (1:20) and DNP-KSHV at a concentration of 1000 viral genomes/cell to be able to saturate the virus binding sites, as described previously [32]. The cells were washed, fixed again, blocked, and treated with goat anti-mouse G20 gold conjugate followed by HRP-coupled donkey anti-goat IgG and TSA-488. Due to the particle size, the 20 nm gold conjugate is excluded from the intracellular compartment and detects only cell surface bound anti-β3 antibody. Residual peroxidase activity was blocked with 1% H2O2. Bound DNP-KSHV was detected with monoclonal rat anti-DNP diluted 1:100 in blotto/NGS followed by goat anti-rat IgG-HRP (1:100) and TSA 647. The nuclei were stained with ethidium homodimer. For quantitation of αVβ3 expression on mitotic and interphase cells, 17 micrograph fields were analyzed. The αVβ3 fluorescence for the individual mitotic cells with metaphase chromosomes was quantitated in each field using the Zeiss LSM software histogram function, as above, and the mean was determined (n = 1-5 cells/field; 42 cells). The αVβ3 fluorescence for the interphase cells was determined by subtracting the mitotic cell fluorescence from the total fluorescence for each of the 17 fields. The number of interphase cells in each field was quantitated and the mean of the αVβ3 pixels/cell was determined (n = 12-35 cells/field; 413 cells). The fluorescent intensity range was from 50-255.
For colocalization of DNP-KSHV and various cell surface receptors in live cell assays, viable HT1080 cells were brought to 4°C and incubated for 1.5 h with various primary antibodies mixed with DNP-KSHV (as above). The primary antibodies recognized αVβ3 (B3A), α3β1, αVβ5 integrins, CD98, and caveolin. The cultures were washed, fixed, and the primary antibodies were detected with anti-mouse IgG-HRP and TSA 488. We also tested the binding of biotinylated cholera toxin B using goat anti-biotin-HRP and TSA 488. In live cell assays, the primary antibodies only detect receptors exposed at the cell surface. DNP-KSHV was then localized as above.
Control experiments were performed to evaluate antibody cross-reactivity and the specificity of sequential TSA amplification steps. HT1080 cells were fixed and incubated simultaneously with DNP-KSHV and non-immune mouse IgG or DNP-mock virus gradient fraction and mouse anti-β3 integrin antibody. Bound mouse antibodies were detected as above with TSA 488 enhancement. Residual peroxidase activity was blocked and bound DNP-KSHV or DNP-mock fraction was detected with a rat anti-DNP as above with TSA 647 enhancement.
For the colocalization of HS and KSHV, fixed HT1080 cells were incubated with DNP-KSHV for 16 h. The cells with bound virus were fixed, blocked, and incubated with mouse anti-HS (10E4) diluted 1:50, followed by goat anti-mouse IgG-HRP and TSA 488. DNP-KSHV was then localized, as above, with TSA 647. The nuclei were stained with ethidium homodimer (Invitrogen). Cell borders were determined using both phase contrast and fluorescent images that were magnified 400X to accurately identify and mark the individual peripheral cell margins. To quantify the HS and KSHV colocalization, the number of fluorescent pixels within a 65 μm2 area over the ethidium stained nuclei was determined using the LSM version 4.2 software. The supranuclear region was selected to ensure that no fluorescent pixels from adjacent cells were recorded.
Confocal microscopy
Confocal images were generated on an LSM 5 Pascal system (Zeiss) equipped with 40× 1.3 NA and 63× 1.4 NA objectives. All figures were maximum projections except Figures 3E and 10. Image maximum projections were compiled from Z-stacks of 30-50 sections with intervals ranging from 0.3-0.4 μm and a Z distance of 10-14μm. Confocal images for all figures, except Figure 10, were deconvolved using AutoQuant X AutoDeblur Gold CF (Media Cybernetics, Inc.). Manders colocalization coefficients were determined using unbiased automatic thresholding (Imaris 7.3) on deconvolved Z-stack images, and reported in the value range 0 1 (0: no colocalization, 1: all pixels colocalize) directly from the software analysis. Three-dimensional surface renderings of deconvolved images were done with Imaris 7.3 (Bitplane AG).
Data analysis
The GraphPad Prism software was used for statistical analysis. The unpaired t test (two-tailed) was used to compare fluorescent pixel counts for the different cell populations.
Research Highlights.
KSHV receptors were characterized using TSA-enhanced confocal microscopy
Integrins αVβ3 and α3β1 were detected on essentially all KSHV-binding domains
KSHV-binding to cell surface domains was not dependent on heparan sulfate
Salivary gland epithelial (HSG) cells lacking αVβ3 are resistant to KSHV infection
Reconstitution of αVβ3 renders HSG cells highly susceptible to KSHV infection
ACKNOWLEDGMENTS
We acknowledge F. Jenkins for the rabbit anti-xCT (P218-232) antiserum, K. Izutsu for the HSG cell line, W. Carter for the HT1080 cell line, M. Ginsberg for the pcDNA3-β3 expression plasmid. This work was supported by the National Institute for Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) under awards number DEO18927 and DE021954 to T. Rose. L.K.D. was supported by a NIH training grant from the University of Washington STD/AIDS Research Training Program T32AI007140-34. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burckhardt CJ, Greber UF. Virus movements on the plasma membrane support infection and transmission between cells. PLoS Pathog. 2009;5:e1000621. doi: 10.1371/journal.ppat.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmuth JA, Burckhardt CJ, Koumoutsakos P, Greber UF, Sbalzarini IF. A novel supervised trajectory segmentation algorithm identifies distinct types of human adenovirus motion in host cells. J Struct Biol. 2007;159:347–358. doi: 10.1016/j.jsb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, et al. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe. 2011;10:105–117. doi: 10.1016/j.chom.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schelhaas M, Ewers H, Rajamaki ML, Day PM, Schiller JT, et al. Human papillomavirus type 16 entry: retrograde cell surface transport along actin rich protrusions. PLoS Pathog. 2008;4:e1000148. doi: 10.1371/journal.ppat.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 10.Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Sattentau Q. Avoiding the void: cell to cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Thorp SC. Cell surface heparan sulfate and its roles in assisting viral infections. Med Res Rev. 2002;22:1–25. doi: 10.1002/med.1026. [DOI] [PubMed] [Google Scholar]
- 13.Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet A, Haumont M, Chellun D, Massaer M, Tufaro F, et al. The varicella zoster virus glycoprotein B (gB) plays a role in virus binding to cell surface heparan sulfate proteoglycans. Virus Res. 1998;53:197–207. doi: 10.1016/s0168-1702(97)00149-4. [DOI] [PubMed] [Google Scholar]
- 15.Akula SM, Pramod NP, Wang FZ, Chandran B. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virol. 2001;284:235–249. doi: 10.1006/viro.2001.0921. [DOI] [PubMed] [Google Scholar]
- 16.Ganem D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol. 2006;1:273–296. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- 17.Birkmann A, Mahr K, Ensser A, Yaguboglu S, Titgemeyer F, et al. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J Virol. 2001;75:11583–11593. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang FZ, Akula SM, Pramod NP, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J Virol. 2001;75:7517–7527. doi: 10.1128/JVI.75.16.7517-7527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn A, Birkmann A, Wies E, Dorer D, Mahr K, et al. Kaposi’s sarcoma-associated herpesvirus gH/gL: glycoprotein export and interaction with cellular receptors. J Virol. 2009;83:396–407. doi: 10.1128/JVI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark L, Lee WH, Spiller OB, Villoutreix BO, Blom AM. The Kaposi’s sarcoma-associated herpesvirus complement control protein (KCP) binds to heparin and cell surfaces via positively charged amino acids in CCP1-2. Mol Immunol. 2006;43:1665–1675. doi: 10.1016/j.molimm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Greene W, Gao SJ. Actin dynamics regulate multiple endosomal steps during Kaposi’s sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 2009;5:e1000512. doi: 10.1371/journal.ppat.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi’s sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol. 2009;83:4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, et al. DC SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 25.Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, et al. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC SIGN. J Virol. 2008;82:4793–4806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaleeba JA, Berger EA. Kaposi’s sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science. 2006;311:1921–1924. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- 27.Veettil MV, Sadagopan S, Sharma Walia N, Wang FZ, Raghu H, et al. Kaposi’s sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol. 2008;82:12126–12144. doi: 10.1128/JVI.01146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. Nat Med. 2012;18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 30.Garrigues HJ, Rubinchikova YE, Dipersio CM, Rose TM. Integrin alphaVbeta3 Binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J Virol. 2008;82:1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akula SM, Wang FZ, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology. 2001;282:245–255. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- 32.Garrigues HJ, Rubinchikova YE, Rose TM. KSHV cell attachment sites revealed by ultra sensitive tyramide signal amplification (TSA) localize to membrane microdomains that are upregulated on mitotic cells. Virol. 2014;452:75–85. doi: 10.1016/j.virol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anilkumar N, Bhattacharya AK, Manogaran PS, Pande G. Modulation of alpha 5 beta 1 and alpha V beta 3 integrins on the cell surface during mitosis. J Cell Biochem. 1996;61:338–349. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C338::AID-JCB2%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Adang LA, Parsons CH, Kedes DH. Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high throughput single-cell analysis of Kaposi’s sarcoma-associated herpesvirus infection. J Virol. 2006;80:10073–10082. doi: 10.1128/JVI.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 36.Wolf AA, Fujinaga Y, Lencer WI. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J Biol Chem. 2002;277:16249–16256. doi: 10.1074/jbc.M109834200. [DOI] [PubMed] [Google Scholar]
- 37.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 38.Lories V, Cassiman JJ, Van den Berghe H, David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Biol Chem. 1989;264:7009–7016. [PubMed] [Google Scholar]
- 39.Shirasuna K, Sato M, Miyazaki T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 1981;48:745–752. doi: 10.1002/1097-0142(19810801)48:3<745::aid-cncr2820480314>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Kerur N, Veettil MV, Sharma Walia N, Sadagopan S, Bottero V, et al. Characterization of entry and infection of monocytic THP 1 cells by Kaposi’s sarcoma-associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology. 2010;406:103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faye C, Moreau C, Chautard E, Jetne R, Fukai N, et al. Molecular interplay between endostatin, integrins, and heparan sulfate. J Biol Chem. 2009;284:22029–22040. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vorup-Jensen T, Chi L, Gjelstrup LC, Jensen UB, Jewett CA, et al. Binding between the integrin alphaXbeta2 (CD11c/CD18) and heparin. J Biol Chem. 2007;282:30869–30877. doi: 10.1074/jbc.M706114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao C, Boylan B, Fang J, Wilcox DA, Newman DK, et al. Heparin promotes platelet responsiveness by potentiating alphaIIbbeta3-mediated outside-in signaling. Blood. 2011;117:4946–4952. doi: 10.1182/blood-2010-09-307751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medeiros VP, Paredes-Gamero EJ, Monteiro HP, Rocha HA, Trindade ES, et al. Heparin-integrin interaction in endothelial cells: downstream signaling and heparan sulfate expression. J Cell Physiol. 2012;227:2740–2749. doi: 10.1002/jcp.23018. [DOI] [PubMed] [Google Scholar]
- 45.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, et al. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203:166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 46.Wickstrom SA, Alitalo K, Keski-Oja J. Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of RhoA activity. J Biol Chem. 2003;278:37895–37901. doi: 10.1074/jbc.M303569200. [DOI] [PubMed] [Google Scholar]
- 47.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang FZ, Akula SM, Sharma-Walia N, Zeng L, Chandran B. Human Herpesvirus 8 Envelope Glycoprotein B Mediates Cell Adhesion via Its RGD Sequence. J Virol. 2003;77:3131–3147. doi: 10.1128/JVI.77.5.3131-3147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan HH, Sharma-Walia N, Streblow DN, Naranatt PP, Chandran B. Focal adhesion kinase is critical for entry of Kaposi’s sarcoma-associated herpesvirus into target cells. J Virol. 2006;80:1167–1180. doi: 10.1128/JVI.80.3.1167-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiMaio TA, Gutierrez KD, Lagunoff M. Latent KSHV infection of endothelial cells induces integrin beta3 to activate angiogenic phenotypes. PLoS Pathog. 2011;7:e1002424. doi: 10.1371/journal.ppat.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demaster LK, Rose TM. A critical Sp1 element in the rhesus rhadinovirus (RRV) Rta promoter confers high-level activity that correlates with cellular permissivity for viral replication. Virology. 2014;448C:196–209. doi: 10.1016/j.virol.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, Zhou F, Greene W, Gao SJ. Rhesus rhadinovirus infection of rhesus fibroblasts occurs through clathrin-mediated endocytosis. J Virol. 2010;84:11709–11717. doi: 10.1128/JVI.01429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarousse N, Chandran B, Coscoy L. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi’s sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J Virol. 2008;82:12591–12597. doi: 10.1128/JVI.01167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxfield SR, Moulder K, Koning F, Elbe A, Stingl G, et al. Murine T cells express a cell surface receptor for multiple extracellular matrix proteins. Identification and characterization with monoclonal antibodies. J Exp Med. 1989;169:2173–2190. doi: 10.1084/jem.169.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroeder N, Chung CS, Chen CH, Liao CL, Chang W. The lipid raft-associated protein CD98 is required for vaccinia virus endocytosis. J Virol. 2012;86:4868–4882. doi: 10.1128/JVI.06610-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, et al. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prager GW, Feral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007;282:24477–24484. doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 59.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 60.Critchley DR, Holt MR, Barry ST, Priddle H, Hemmings L, et al. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem Soc Symp. 1999;65:79–99. [PubMed] [Google Scholar]
- 61.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, et al. Integrin alpha3beta1, a novel receptor for alpha3(IV) noncollagenous domain and a trans-dominant Inhibitor for integrin alphavbeta3. J Biol Chem. 2006;281:20932–20939. doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- 63.Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, Hynes RO. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JP, Zhang K, Kramer RH, Schall TJ, Woodley DT. Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of alpha 3 beta 1 epiligrin receptor. J Invest Dermatol. 1992;98:764–770. doi: 10.1111/1523-1747.ep12499947. [DOI] [PubMed] [Google Scholar]
- 65.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty S, Veettil MV, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. Proc Natl Acad Sci U S A. 2012;109:E1163–1172. doi: 10.1073/pnas.1119592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 68.Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, et al. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 70.Krutzsch HC, Choe BJ, Sipes JM, Guo N, Roberts DD. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J Biol Chem. 1999;274:24080–24086. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- 71.Nishiuchi R, Murayama O, Fujiwara H, Gu J, Kawakami T, et al. Characterization of the ligand-binding specificities of integrin alpha3beta1 and alpha6beta1 using a panel of purified laminin isoforms containing distinct alpha chains. J Biochem (Tokyo) 2003;134:497–504. doi: 10.1093/jb/mvg185. [DOI] [PubMed] [Google Scholar]
- 72.Wu C, Chung AE, McDonald JA. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108:2511–2523. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]
- 73.Chakraborty S, ValiyaVeettil M, Sadagopan S, Paudel N, Chandran B. c-Cbl-mediated selective virus-receptor translocations into lipid rafts regulate productive Kaposi’s sarcoma-associated herpesvirus infection in endothelial cells. J Virol. 2011;85:12410–12430. doi: 10.1128/JVI.05953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, et al. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]