Abstract
We reported previously that even though immunization with the recombinant mycobacterial 27-kDa lipoprotein (r27) induced a Th1-type response in mice, the vaccinated mice became more susceptible to challenge with Mycobacterium tuberculosis. In this study we show that r27 stimulates naive splenocytes to proliferate. Acylation of r27 was crucial for this effect, since a nonacylated mutant of r27, termed r27ΔSP, failed to stimulate splenocytes either in vitro or in vivo. Depletion experiments indicated that only B cells were proliferating in a T-cell-independent manner. We also found that r27 is recognized by TLR2, which is involved in mitogenic stimulation. Interestingly, r27 but not r27ΔSP induced high gamma interferon levels in splenocyte supernatants, whereas no significant interleukin-2 levels were detected. Since B-cell polyclonal activation might aggravate pathogen infection, we asked whether the antiprotective effect of the r27 lipoprotein is associated with its mitogenicity. We showed that, as in the case of r27, immunization of mice with the nonmitogenic r27ΔSP lipoprotein resulted in increased M. tuberculosis multiplication. We conclude that the antiprotective effect of the r27 lipoprotein must be linked to properties of the polypeptide portion of the lipoprotein rather than to its lipid moiety and its mitogenicity.
The 27-kDa antigen, the product of the Mycobacterium tuberculosis lprG gene, is a putative lipoprotein that is present exclusively in the membrane of the M. tuberculosis species complex (5). Previously, it was reported that mice immunized with r27 were more susceptible to M. tuberculosis challenge than nonimmunized mice (15). Splenic CFU counts in mice immunized with the recombinant mycobacterial 27-kDa lipoprotein (r27) were significantly higher, by about 0.5 to 0.8 log unit, than those in nonimmunized mice. Furthermore, the protection afforded following immunization with the Mycobacterium bovis BCG vaccine was completely abolished when r27 was added to the BCG vaccine (15). These results were unexpected, because r27 induces a typical Th1-type immune response thought to be crucial for protection against intracellular pathogens such as M. tuberculosis. It was found recently that M. tuberculosis with a knockout of the 27-kDa (lprG) gene has an attenuated virulence phenotype in mice (A. Cataldi, personal communication). This finding supports our previous results and indicates that the native 27-kDa lipoprotein might also have a role in enhancing M. tuberculosis infection.
Lipoproteins have been reported to be powerful antigens that induce strong antibody- and cell-mediated immune responses (7, 10). The lipid moiety on mature acylated lipoproteins or synthetic acylation of peptides increased their immunogenicity and induced their adjuvant-like properties (4). Bacterial lipoproteins are recognized by the innate immune system through toll-like receptor 2 (TLR2) together with TLR1 or TLR6, all of which induce antibacterial activity in macrophages (27-29). These findings indicate that lipoproteins might be promising candidates for vaccination. However, lipoproteins have also been shown to induce strong polyclonal B-cell activation, as reported for Borrelia burgdorferi (13), Escherichia coli (21), and Salmonella enterica serovar Typhimurium (16), and a similar activity has been shown by a synthetic lipopeptide analog that mimics the N-terminal end of the E. coli outer membrane lipoprotein (17). Polyclonal stimulation of the immune response could be a major obstacle in the design of vaccines against infectious agents, since immunization with mitogens can suppress the specific immune response required for protection (24).
In this study we show that r27 is a T-cell-independent B-cell mitogen and that its activation is mediated by TLR2. Since mitogens have been reported to interfere with the protective immune responses following immunization, we investigated whether the mitogenicity of r27 is responsible for the deleterious effect provoked by this antigen in M. tuberculosis vaccines.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female BALB/c, BALB/c-nu/nu, C3H/HeJ, C3HEB/FeJ, and C3H/HeN mice, aged 5 to 6 weeks, were purchased from Harlan Biotech Israel (Rehovot, Israel). Animals were maintained under specific-pathogen-free conditions during the experiments.
Reagents.
RPMI 1640 medium, Ham's F-12 medium, Dulbecco's modified Eagle medium (DMEM), trypsin-EDTA, HEPES, glutamine, and the penicillin-streptomycin-nystatin antibiotic mix were from Biological Industries (Kibbutz Beit Haemek, Israel). Hygromycin B and G418 sulfate were purchased from Calbiochem (San Diego, Calif.), and ciprofloxacin was purchased from Bayer AG (Leverkusen, Germany). The fluorescein isothiocyanate-conjugated rat anti human CD25 antibody was obtained from Serotec (Oxford, United Kingdom). Polymyxin B, lipopolysaccharide (LPS), and concanavalin A were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell lines.
The RAW 264.7 (American Type Culture Collection) cell line was grown in DMEM supplemented with 10% fetal calf serum, 1 mM glutamine, 25 mM HEPES, penicillin and streptomycin (both at 100 μg/ml), and nystatin (12.5 U/ml). Chinese hamster ovary (CHO) reporter cells were a gift from D. T. Golenbock. These cells are stably transfected with a plasmid encoding the NF-κB-controlled surface expression of human CD25. In addition to the reporter construct, clone 3E10 expresses human CD14 (CHO/CD14) and clone 3E10/TLR2 expresses human CD14 and TLR2 (CHO/CD14/TLR2) (19). CHO/CD14 cells were grown in Ham's F-12 medium supplemented with 10% fetal bovine serum with the addition of 400 U of hygromycin B/ml and 100 μg of ciprofloxacin/ml. G418 (0.5 mg/ml) was also added to CHO/CD14/TLR2.
Cloning and protein purification.
r27 was cloned as described previously (15). In order to create the nonacylated 27-kDa mutant, we cloned the lprG gene starting from the serine codon at position 28. The mutant lprG gene was inserted into the pQE9 vector (Qiagen, Hilden, Germany), which added a histidine tag to the N terminus of the expressed protein. The primers contained SalI and HindIII restriction sites, and the sequences were 5′-ACGCGTCGACTCGTCGGGCTCGAAGCC and 3′-CCCAAGCTTGCTCACCGGGGGCTTCG. Sequences of the r27 and r27ΔSP clones were confirmed by automated sequencing (Perkin-Elmer Applied Biosystems). r27 and the nonacylated protein r27ΔSP were purified as previously described (15), and their sequences were confirmed by Edman degradation and by mass spectrometry with Qtof2 (Micromass, Manchester, England). The purified proteins were dialyzed against saline and then transferred to a column containing immobilized polymyxin B to remove contaminant LPS. The amount of LPS in the protein fraction was measured quantitatively with the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.) and found to be <0.01 endotoxin unit (EU) per μg of antigen.
Proliferation assay.
Naive mice were sacrificed, and splenocytes were aseptically harvested for the proliferation assay. Red blood cells were lysed by addition of ACK medium (0.15 M NH4Cl, 1.0 mM KHOC3, and 0.1 mM disodium EDTA), and lymphocytes were pooled and grown in 96-well plates (Nunc, Roskilde, Denmark) at a concentration of 4 × 105 cells/well. RPMI 1640 medium supplemented as described for DMEM was used for in vitro cultures. Splenocytes were restimulated with 10 μg of r27/ml, 5 μg of LPS or concanavalin A/ml. In some experiments, polymyxin B (5 μg/ml) was added to the antigen and then incubated for 1 h at 37°C before the mixture was added to the splenocytes. After 24 h of incubation, splenocytes were pulsed with 0.5 μCi of [methyl-3H]thymidine/well (25 Ci/mmol; Amersham Pharmacia Biotec); they were harvested 16 h later with a cell harvester, and incorporated [methyl-3H]thymidine counts were determined. In some experiments, prior to r27 addition, splenocytes were cultured for 24 h with a phosphorothioate-modified antisense oligonucleotide (5′-GACCGCCTGCCCGGAGCCTAGG-3′) or sense oligonucleotide (5′-CCTAGGCTCCGGGCAGGCGGTC-3′), specific for the mouse TLR2 gene (5 μM). We defined the stimulation index as the counts per minute obtained from cells stimulated by the antigen divided by the counts per minute obtained from cells without antigen stimulation.
Cytokine assays.
Secretions of gamma interferon (IFN-γ) and interleukin 10 (IL-10) in supernatants of normal splenocytes were monitored after 48 h of stimulation as described above. Splenocytes from immunized mice (4 × 105 cells/well) were stimulated in 96-well plates with r27 or r27ΔSP (5 μg/ml) for 96 h, and then supernatants were collected. Cytokine levels were determined by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions, by using commercial pairs of antibodies and recombinant cytokines (Pharmingen International, San Diego, Calif.) as previously described (15).
T- and B-cell purification.
StemSep kits (StemCell Technologies Inc., Vancouver, British Columbia, Canada) were used for B- and T-cell preparations according to the manufacturer's instructions. In some experiments the purified T cells underwent an additional purification cycle to increase their purity. The purities of T and B cells were tested by fluorescence-activated cell sorting (FACS) and found to be >90 to 98%. After purification, proliferation assays were performed as described above. In some experiments, purified T cells were labeled with 5 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE) before exposure to r27, and proliferation was analyzed by flow cytometry (FACScan; Becton Dickinson).
IL-12 secretion on RAW 264.7 and peritoneal macrophages.
RAW 264.7 cells (5 × 105 cells/well) were stimulated for 24 h with various concentrations of r27 or r27ΔSP in the presence of polymyxin B, and supernatants were collected. In another experiment, thioglycolate-elicited peritoneal macrophages from C3HEB/FeJ and C3H/HeJ mice were stimulated with LPS (10 ng/ml) and r27 (10 μg/ml), and supernatants were collected after 24 h. IL-12 secretion was measured by ELISA (Pharmingen International).
TLR2 activation.
The CHO/CD14 and CHO/CD14/TLR2 cell lines were plated in 24-well plates at 2.5 × 105 cells/well for 18 h. After this period, different concentrations of r27 or r27ΔSP were added to the cells, and the plates were incubated for an additional 24 h. Subsequently, the cells were harvested with trypsin-EDTA and labeled with rat anti-human CD25 in phosphate-buffered saline with 0.5% bovine serum albumin for 40 min. The cells were analyzed by flow cytometry (FACScan; Becton Dickinson).
RNA purification and semiquantitative RT-PCR.
Splenocytes were prepared and grown as described above. RNA was prepared with TRI reagent (Sigma Chemical Co.) according to the manufacturer's instructions. After purification, RNA samples were treated with RNase-free DNase I (Ambion, Huntingdon, Cambridgeshire, United Kingdom) for 1 h at 37°C, followed by phenol-chloroform extraction. Reverse transcription-PCR (RT-PCR) was performed with the Access RT-PCR system (Promega, Madison, Wis.) by using the TLR2-specific primers TLR2-F (GGAGCGGCTGCAGGACTC) and TLR2-R (CCAAAGAGCTCGTAGCATCC). As a control we used the following primers for the β-actin gene: β-actin-F (TGGAATCCTGTGGCATCCATGAAAC) and β-actin-R (TAAAACGCAGCTCAGTAACAGTCCG). RT-PCR products were analyzed by agarose DNA electrophoresis, and band intensities were quantified by using TINA software.
Mitogenicity of the 27-kDa antigen in vivo.
To test the mitogenicity of the r27 protein in vivo, BALB/c mice were intravenously injected with 25 μg of r27 or with the r27ΔSP mutant. After 3 days, the spleens were removed and weighed, and splenocytes were counted by trypan blue exclusion.
Immunization and infection with M. tuberculosis.
BALB/c mice were immunized twice, at 2-week intervals, with 10 μg of r27 or r27ΔSP, emulsified or not in Ribi adjuvant (MPL + TDM emulsion; Sigma Chemical Co.). Three weeks after the last immunization, sera were collected from the mice, and titers of antibody against the relevant immunizing antigen were analyzed by ELISA as previously described (15). Four weeks after the last immunization, BALB/c mice were infected intravenously with 5 × 105 CFU of M. tuberculosis strain H37Rv (a gift from Gilles Marchal). Five weeks after the challenge, mice were sacrificed, spleens were homogenized in saline, and serial dilutions were plated onto 7H9 agar plates for counting of M. tuberculosis CFU. For delayed-type hypersensitivity (DTH) analysis, mice were infected with M. tuberculosis for 3 months and then injected in the right footpad with 10 μg of r27 or r27ΔSP in 20 μl of saline. At the same time, saline was injected into the left footpad, and local swelling in each footpad was measured after 24 h with a micrometer (Mitutoyo, Aurora, Japan).
Ethical considerations.
All experiments were performed in accordance with the regulations of the animal experimentation ethics committee of the Hebrew University—Hadassah Medical School.
Statistical analysis.
Data were analyzed by Student's t test. P values of <0.05 were considered significant. Results shown are means ± standard deviations. Proliferation and cytokine determinations were carried out three to five times on pooled samples from three mice. DTH responses for each protein were measured twice in groups of five mice. Immunization and challenge experiments were performed two to three times, with 10 mice in each group.
RESULTS
Murine lymphocytes proliferate in response to stimulation with r27.
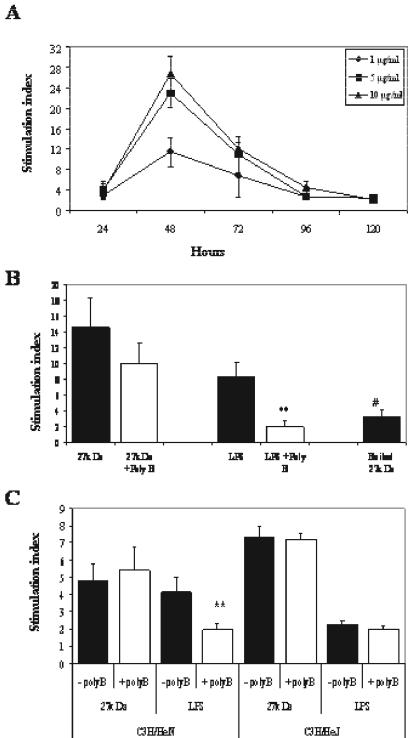
Naive splenocytes were incubated with various concentrations of r27, and incorporation of [3H]TdR was determined at several time points to measure proliferation. Proliferation was found to be dose and time dependent. The highest proliferation was found after 48 h (Fig. 1A), and the submitogenic dose was 0.2 μg/ml (data not shown). Since r27 was purified from E. coli, we excluded LPS contamination by showing that preincubation of r27 with polymyxin B did not influence proliferation (Fig. 1B). Denaturation of r27 by boiling diminished the proliferative response, confirming that the proliferation was induced by r27 and not by LPS contamination (Fig. 1B). Finally, splenocytes from LPS-hyporesponsive C3H/HeJ mice proliferated in response to simulation with r27, to the same degree as the genetically related LPS-responsive C3H/HeN strain (Fig. 1C).
FIG. 1.
Proliferative response of naive BALB/c splenocytes to r27. (A) BALB/c splenocytes (4 × 105/well) were stimulated with various concentrations of r27. [3H]thymidine was added at different time points, and proliferation was measured after 16 h of incubation. (B and C) BALB/c splenocytes (B) or C3H/HeN and C3H/HeJ splenocytes (4 × 105/well) (C) were stimulated with either r27 (10 μg/ml), LPS (5 μg/ml), or boiled r27 (10 μg/ml) for 24 h in the presence or absence of polymyxin B (5 μg/ml). After this period, [3H]thymidine was added for 16 h, and the proliferation response was measured. **, P < 0.001 (compared to stimulation with LPS and without polymyxin B); #, P < 0.001 (compared to stimulation with r27).
Acylation of the 27-kDa lipoprotein is crucial for proliferation.
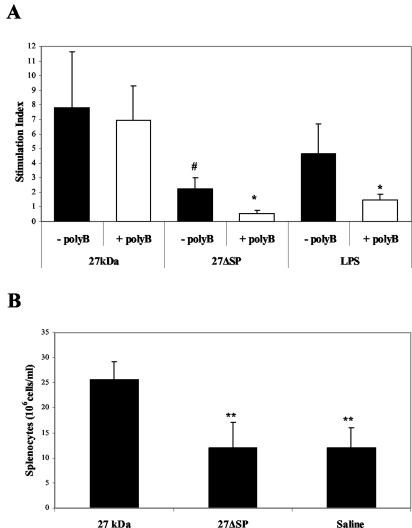
To examine if the acylation of r27 is important for lymphocyte proliferation, we created a nonacylated 27-kDa mutant. In this mutant, named r27ΔSP, the N terminus comprising the signal peptide and a cysteine at position 27 were replaced with a six-histidine tail. Attempts to replace the cysteine with alanine or glycine while the histidine tail is added to the C terminus (as in r27) failed to produce a recombinant product, indicating a possible link between translation and acylation. The absence of acylation in r27ΔSP was confirmed by mass spectrometry and by metabolic labeling with [3H]palmitate (data not shown). As shown in Fig. 2A, addition of r27ΔSP to splenocyte cultures induced minor proliferation compared to that with r27, revealing that acylation is crucial for splenocyte stimulation. Moreover, this weak proliferation was eliminated by the addition of polymyxin B, indicating that this proliferation was induced by a negligible amount of LPS contaminating the protein sample. Intravenous injection of r27 into mice resulted in significant splenomegaly compared to the control group (P < 0.001), whereas no effect was found after injection of r27ΔSP (Fig. 2B). These results indicate that acylation is crucial for mitogenic stimulation both in vitro and in vivo.
FIG. 2.
Acylation of r27 is crucial for its mitogenicity. (A) BALB/c splenocytes (4 × 105/well) were stimulated with r27, r27ΔSP (10 μg/ml), or LPS (5 μg/ml) for 24 h in the presence or absence of polymyxin B (5 μg/ml). After this period, [3H]thymidine was added for 16 h, and the proliferation response was measured. #, P < 0.001 (compared to stimulation with r27); *, P < 0.01 (compared to the relevant non-polymyxin B-treated group). (B) Mice were injected intravenously with 25 μg of r27, r27ΔSP, or saline as a control. Five days after the injection, spleens were removed, and total splenocytes were counted by trypan blue exclusion. **, P < 0.001 (compared to r27-injected mice).
T cells are not required for B-cell proliferation.
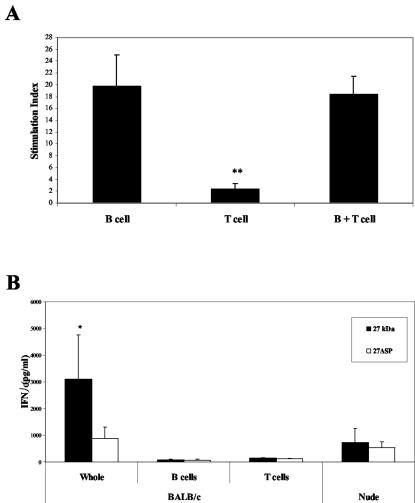
Murine spleen cells are composed mainly of B cells (55%) and T cells (35%). In order to identify which type of cells responded to r27, we purified B or T cells from splenocytes of BALB/c mice. The data reported in Fig. 3A demonstrate that only B cells proliferated in response to r27 and that the presence of T cells was not required for this stimulation. Surprisingly, high IFN-γ secretion levels were found in splenocyte cultures after stimulation with r27 but not with r27ΔSP (Fig. 3B,) whereas no significant IL-2 levels were found in either case. It is well established that IFN-γ is secreted mostly by T cells or natural killer cells (NK cells). However in our experiments (Fig. 3B), no secretion was found in the purified B and T cells; therefore, we tested if NK cells were involved in the IFN-γ secretion. Stimulation of splenocytes from nu/nu mice (a T-cell-deficient mouse strain) with r27 did not result in significant levels of IFN-γ production. These results imply that T cells might be involved in IFN-γ secretion, yet this activity probably requires the presence of other cells such as macrophages that are missing in the purified T-cell preparations.
FIG. 3.
B cells proliferate in response to r27. (A) B or T cells were purified from BALB/c splenocytes and stimulated with r27 (10 μg/ml) for 24 h; then [3H]thymidine was added for an additional 16 h. **, P < 0.001 (compared to B cells). (B) IFN-γ levels were measured after 48 h of stimulation with r27 in whole BALB/c splenocytes, purified B or T cells, and whole nu/nu splenocytes. *, P < 0.01 (compared to stimulation with r27ΔSP).
Splenocyte proliferation is TLR2 dependent.
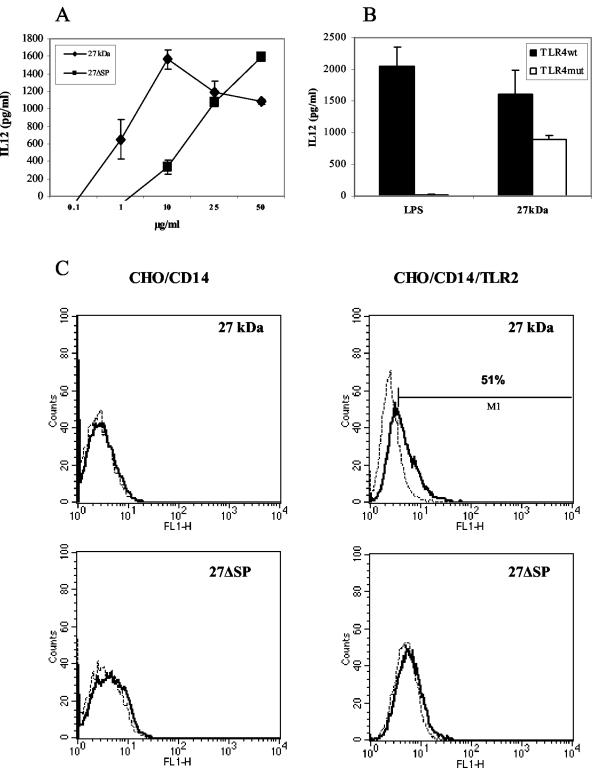
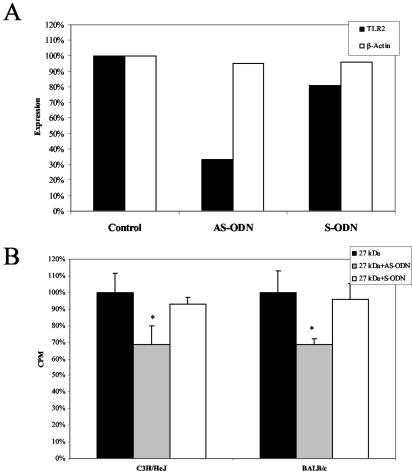
Several studies have identified lipoproteins as TLR2 ligands (29). Therefore, we tested the ability of r27 to activate this receptor. We found that r27 is able to induce IL-12 secretion in RAW 264.7 cells, and the presence of polymyxin B did not influence this secretion (Fig. 4A). The nonacylated r27ΔSP mutant induced IL-12 secretion as well, but at levels almost five times lower than those induced by the acylated form. IL-12 secretion was found also in peritoneal macrophages from the TLR4 mutant strain, C3H/HeJ, stimulated with r27 (Fig. 4B). These results demonstrate that r27 activates macrophages through an innate receptor other than TLR4. To confirm the TLR2 activity of r27, we used CHO cells stably transfected with a reporter plasmid containing NF-κB-dependent surface expression of human CD25. In order to respond to TLR2 ligands, CHO cells require CD14 and TLR2. We tested reporter cells stably transfected with CD14 alone (CHO/CD14) or with CD14 and TLR2 (CHO/CD14/TLR2) (a kind gift from D. Golenbock) (19). We found that 52% of the CHO/CD14/TLR2 cells expressed the CD25 marker when stimulated with r27, whereas negligible expression (8%) was found in CHO/CD14 cells (Fig. 4C), demonstrating that r27 specifically activates TLR2. We also found that r27ΔSP failed to induce CD25 expression at the protein concentrations tested; however, at much higher concentrations (25 to 50 μg/ml), r27ΔSP activated TLR2-expressing cells (data not shown). In order to determine whether splenocyte stimulation depends on TLR2 activation, we incubated the splenocytes with antisense (TLR2-AS) or sense (TLR2-S) oligonucleotides against TLR2. Semiquantitative RT-PCR analysis showed that TLR2 mRNA levels were lowest when splenocytes were treated with TLR2-AS (67% inhibition) (Fig. 5A). Accordingly, we found a significant decrease in splenocyte proliferation when TLR2-AS was added (35 to 40%; P < 0.001). In contrast, no significant difference was found between the TLR2-S-treated group and control splenocytes (Fig. 5B). Therefore, we conclude that TLR2 activation by r27 is important for mitogenic stimulation.
FIG. 4.
r27 is recognized by TLR2. (A) RAW 264.7 cells (5 × 105/well) were stimulated with various concentration of r27 or r27ΔSP in the presence of polymyxin B. After 24 h, culture supernatants were collected from the wells, and IL-12 levels were measured by ELISA. (B) Thioglycolate-elicited peritoneal macrophages from C3HEB/FeJ and C3H/HeJ mice were stimulated with LPS (10 ng/ml) or r27 (10 μg/ml), supernatants were collected after 24 h, and IL-12 secretion was measured by ELISA. (C) CHO/CD14 and CHO/CD14/TLR2 cells (5 × 105/well) were stimulated with r27 or r27ΔSP (5 μg/ml) for 24 h. After this period, cells were collected, labeled with fluorescein isothiocyanate-conjugated anti-human CD25, and then analyzed by FACS. Similar results were obtained in four independent experiments.
FIG. 5.
Splenocyte proliferation induced by r27 is TLR2 dependent. BALB/c or C3H/HeJ splenocytes (4 × 105/well) were incubated with TLR2 antisense (AS-ODN) or sense (S-ODN) oligonucleotides (5 μM) for 24 h. (A) After this period, mRNA was prepared from the cells, and TLR2 or β-actin mRNA levels were detected by RT-PCR and quantified by using TINA software. (B) Alternatively, after 24 h, r27 was added to the treated splenocyte cultures for another 24 h; then [3H]thymidine was added for 16 additional h, and the proliferation response was measured. *, P < 0.01 (compared to untreated splenocytes).
Acylation enhances the immune response in mice.
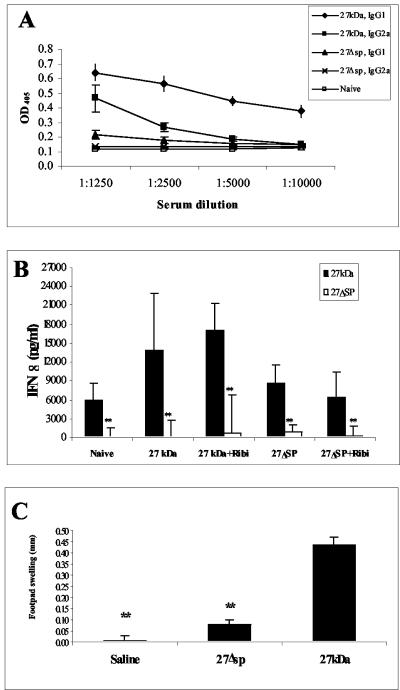
In the experiments described above, we examined the influence of acylation in naive mice. Since the antiprotective effect of r27 had been demonstrated previously on immunized mice (15), we now analyzed the effect of acylation on the immune responses of immunized mice. Mice were injected with r27 or r27ΔSP with or without Ribi adjuvant. As shown in Fig. 6A, antibody titers of both immunoglobulin G1 (IgG1) and IgG2a isotypes against both proteins were notably high in r27-immunized mice, whereas only negligible antibody levels were detected after immunization with r27ΔSP. IFN-γ secretion in splenocyte culture was significantly higher in r27-immunized groups (P < 0.005) (Fig. 6B). Interestingly, no IFN-γ secretion was detected when splenocyte cultures were stimulated with r27ΔSP. Production of nitric oxide was similar to that of IFN-γ, and no significant IL-10 secretion was found in any of the immunized groups (data not shown). These results indicate that acylation enhanced the immunogenicity of the 27-kDa protein and directed it toward the Th1-type response. We next tested the DTH response in M. tuberculosis-infected mice after footpad injection of the proteins. r27 induced a significant DTH response in infected mice (swelling, 0.43 ± 0.06 mm; P < 0.001), whereas only a weak response was found when r27ΔSP was injected (Fig. 6C). These results demonstrate the enhanced immunogenicity afforded by acylation.
FIG. 6.
Immune response induced by the acylated form of the 27-kDa protein. Mice were immunized with either r27 or r27ΔSP, in the presence or absence of Ribi. (A) Antibody titers against r27 or r27ΔSP were measured for immunized mice by using the relevant immunizing antigen. OD405, optical density at 405 nm. (B) IFN-γ secretion in splenocytes from immunized groups after stimulation with r27 or r27ΔSP. (C) DTH response to r27 or to r27ΔSP in M. tuberculosis-infected mice. **, P < 0.005 (compared to stimulation with r27).
Both r27 and r27ΔSP increase CFU numbers after M. tuberculosis challenge.
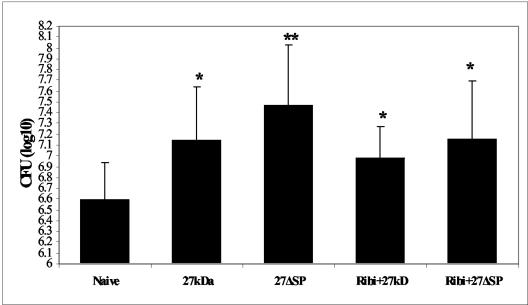
Mitogens cause a broad and nonspecific immune stimulation in the host that might impair the protective immune response against pathogens. Since our results indicate that r27 stimulates naive B-cell proliferation, we asked whether the antiprotective effect engendered by r27 was connected to its mitogenic activity. To answer this question, we immunized mice with either r27 or its nonmitogenic mutant, r27ΔSP, followed by M. tuberculosis challenge (Fig. 7). Our results show that both immunization with r27 and immunization with r27ΔSP resulted in significantly higher M. tuberculosis CFU counts than those for nonimmunized control mice (increases, 0.51 and 0.87 log unit, respectively; P < 0.05 for both comparisons). However, there were no significant differences in CFU between r27- and 27ΔSP-immunized mice. These results indicate that the mitogenicity of the acylated 27-kDa protein is not connected to its deleterious effect on protection. Moreover, emulsifying the proteins with Ribi adjuvant to enhance their immunogenicity did not abolish the antiprotective effect (increases in CFU, 0.38 and 0.53 log unit for r27 and r27ΔSP, respectively; P < 0.05).
FIG. 7.
Both r27 and r27ΔSP increase M. tuberculosis CFU numbers in immunized mice. BALB/c mice were immunized twice with 10 μg of r27 or r27ΔSP 2 weeks apart. After 4 weeks, mice were challenged with 5 × 105 CFU of M. tuberculosis strain H37Rv; 5 weeks later, mice were sacrificed, and CFU in the spleen were determined. This experiment was repeated three times; results of one representative experiment are presented here. *, P < 0.05; **, P < 0.01 (compared to the naive group).
DISCUSSION
In this study we analyzed the mitogenic properties of the recombinant mycobacterial 27-kDa lipoprotein. We showed that r27 induced proliferation in normal splenocytes in a time- and dose-dependent manner. Several lines of evidence showed that splenocyte stimulation was not due to endotoxin contamination. First, preincubation of r27 with polymyxin B, an LPS inhibitor, did not impair splenocyte proliferation. Second, r27 was able to stimulate splenocytes from the LPS nonresponder strain C3H/HeJ. Third, boiling r27 eliminated the mitogenic activity of the lipoprotein. We demonstrated that acylation is crucial for mitogenicity both in vitro and in vivo, since r27ΔSP, the nonacylated mutant, failed to stimulate naive splenocytes and did not induce splenomegaly in mice. Splenocyte depletion experiments revealed that only B cells were stimulated by r27. Such stimulation by lipoprotein and nonlipoprotein molecules has been reported previously (8, 13).
A very interesting finding was the presence of high IFN-γ levels in r27-stimulated splenocyte culture, while no significant IL-2 secretion was observed. This secretion was acylation dependent, as evidenced by the fact that only r27 and not r27ΔSP induced it. Stimulation of purified T cells with r27 did not show any IFN-γ secretion; thus, we tested if B cells secreted IFN-γ. There is evidence that B cells can produce IFN-γ after B-cell-receptor triggering (18), yet we could not detect such secretion in the purified B-cell culture. We next asked if NK cells, which are considered potent IFN-γ producers, are responsible for this secretion. It was shown that preactivated B cells can induce IFN-γ secretion by NK cells via an interaction that requires direct cell contact (22, 31). However, when splenocytes from BALB/c nu/nu mice, a T-cell-deficient strain, were stimulated with r27, no significant IFN-γ secretion was detected. Therefore, although the source of this secretion cannot be clearly defined, it might be that T cells are involved in this secretion, and the presence of other splenic cells, missing in the purified T-cell fraction, may be important to mediate this process. A similar pattern of IFN-γ secretion has also been reported with the alkaline antigen of Trypanosoma cruzi (23). This antigen, like r27, induces T-cell-independent B-cell polyclonal activation; however, the investigators could not determine which cells were responsible for the IFN-γ secretion. The high IFN-γ levels may function to restrict polyclonal lymphocyte stimulation, since it has been demonstrated that IFN-γ regulates the differentiation of stimulated B cells (1).
We showed that r27 induced IL-12 secretion in RAW 264.7 macrophages as well as in peritoneal macrophages from TLR4 mutant mice. These results demonstrate that r27 activates macrophages through an innate receptor other than TLR4, again ruling out nonspecific activation by LPS contamination in our recombinant-protein preparations. Moreover, we showed also that r27 activates human TLR2 in CHO cells transfected with CD14 and TLR2. Thus, our results demonstrate that the 27-kDa lipoprotein is recognized by murine and human TLR2. To date, no discrepancy has been reported between human and mouse TLR2 activation by bacterial lipoproteins. These findings correlate with other observations that identified bacterial lipoproteins as TLR2 ligands (6, 19). Given that the 27-kDa lipoprotein is a TLR2 activator and only the acylated form of r27 succeeded at inducing splenocyte proliferation, it was of interest to clarify whether this proliferation was TLR2 dependent or not. We found significant inhibition of the proliferation response (35 to 40%; P < 0.001) when TLR2 antisense and not TLR2 sense oligonucleotides were added to splenocyte cultures. This finding indicates that the mitogenicity of r27 is connected to the activation of TLR2. Nevertheless, splenocyte proliferation was not completely inhibited by the TLR2 antisense oligonucleotides. Indeed, even though TLR2 mRNA levels were clearly reduced after the antisense treatment, significant amounts of mRNA (33%) still remained in the cells, indicating that TLR2 expression was not totally inhibited. Although the acylation moiety was found to be important for TLR2 activation (11), it was shown recently that the nonacylated form of the M. tuberculosis 19-kDa lipoprotein was able to induce apoptosis through activation of the TLR2 receptor (20). We also found that the nonacylated r27ΔSP mutant activates TLR2, yet this activation was almost five times lower than that with the acylated form. It is possible that the 19- and 27-kDa proteins, both mycobacterial lipoproteins, share an additional motif other than acylation that is recognized by TLR2. Since no mitogenic stimulation was induced by r27ΔSP, even at higher concentrations, additional costimulatory molecules might be required for the mitogenic proliferation of B cells, and acylation is essential for this activation.
Immunization of mice with r27 and its nonacylated form revealed several acylation-dependent differences in the immune response. Antibody production was dramatically lower in r27ΔSP-immunized mice than in mice immunized with r27. Nevertheless, immunization of mice with emulsified r27ΔSP resulted in antibody levels similar to those obtained with the acylated form of r27 without adjuvant (data not shown). It is plausible that the acylation of r27 provides the protein with an adjuvant-like property, as has been reported previously for other proteins (2, 4). It was also shown that this property is related to TLR2 activation (30). Significant levels of IFN-γ secretion and nitric oxide production (data not shown) were found in splenocytes of r27-immunized mice. Interestingly, secretion was detected only when splenocytes were stimulated in vitro with r27, not when they were stimulated with 27ΔSP. Several reports have demonstrated that the lipid moiety enhances antigen presentation in antigen-presenting cells (14, 25). Accordingly, our findings also indicate that acylation of r27 was crucial for in vitro cytokine secretion in splenocytes from immunized mice. Acylation was found to be significant in the DTH response as well: only r27 induced a strong DTH response in M. tuberculosis-infected mice. This result fits earlier observations that acylation enhances the DTH response (3, 9).
We showed that r27 induces B-lymphocyte polyclonal expansion. By inducing such activation, mitogens might interfere with the protective immune response against pathogens (24). A possible connection between B-cell expansion and enhanced virulence has been reported. In Leishmania major infection, it was found that the increase in the B-cell population subsequent to IL-7 administration resulted in exacerbation of the infection (12). In another study, B-cell depletion by continuous anti-IgM injection enhanced the resistance of BALB/c mice to Leishmania tropica and Leishmania mexicana (26). It was also reported that injection of mice with trans-sialidase, a B-cell mitogen, caused a dramatic increase in their susceptibility to T. cruzi infection (8). These studies indicate that immunization of mice with mitogens might have a negative influence on the immune system, exerting an antiprotective effect on the response to subsequent challenge. Thus, we asked if the deleterious effect of r27 previously reported (15) was connected to its mitogenicity. To address this question, we challenged r27- and r27ΔSP-immunized mice with M. tuberculosis. We found that CFU counts in spleens from r27ΔSP- or r27-immunized mice were comparable and significantly higher than CFU counts for nonimmunized mice (P < 0.05). This finding indicates that, in contrast to the studies cited above, the B-cell mitogenic activity of r27 is not linked to its antiprotective effect. It is clear from our data that the polypeptide sequence of r27 rather than the lipid moiety is responsible for its deleterious effect. We do not know as yet how r27 interferes with the natural resistance of BALB/c mice to M. tuberculosis infection. Currently, we are analyzing different peptides of the 27-kDa polypeptide to determine which part is responsible for this deleterious effect.
Identification of molecules such as the 27-kDa lipoprotein is important for understanding the course of M. tuberculosis infection. Such information might help us to develop attenuated M. tuberculosis strains that will produce better protection. In addition, by finding out how r27 aggravates infection, we can develop methods to avoid similar phenomena with other infectious agents.
Acknowledgments
This work was supported by a grant from the Center for the Study of Emerging Diseases (CSED). The studies were performed in the Peter A. Krueger P3 laboratory with the generous financial support of Nancy and Lawrence E. Glick.
We thank Itai Eyal for helpful comments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abed, N. S., J. H. Chace, A. L. Fleming, and J. S. Cowdery. 1994. Interferon-gamma regulation of B lymphocyte differentiation: activation of B cells is a prerequisite for IFN-γ-mediated inhibition of B cell differentiation. Cell. Immunol. 153:356-366. [DOI] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., A. Melhus, C. Capiau, O. van Opstal, and A. Forsgren. 1997. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect. Immun. 65:5010-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrach, G., M. Banai, Y. Fishman, and H. Bercovier. 1997. Delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infect. Immun. 65:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2:425-431. [DOI] [PubMed] [Google Scholar]
- 5.Bigi, F., C. Espitia, A. Alito, M. Zumarraga, M. I. Romano, S. Cravero, and A. Cataldi. 1997. A novel 27 kDa lipoprotein antigen from Mycobacterium bovis. Microbiology 143:3599-3605. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuenkova, M., and M. E. Pereira. 1995. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas' disease. J. Exp. Med. 181:1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coon, J., and R. Hunter. 1975. Properties of conjugated protein immunogens which selectively stimulate delayed-type hypersensitivity. J. Immunol. 114:1518-1522. [PubMed] [Google Scholar]
- 10.Cote-Sierra, J., A. Bredan, C. M. Toldos, B. Stijlemans, L. Brys, P. Cornelis, M. Segovia, P. de Baetselier, and H. Revets. 2002. Bacterial lipoprotein-based vaccines induce tumor necrosis factor-dependent type 1 protective immunity against Leishmania major. Infect. Immun. 70:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 12.Hoerauf, A., W. Solbach, M. Rollinghoff, and A. Gessner. 1995. Effect of IL-7 treatment on Leishmania major-infected BALB.Xid mice: enhanced lymphopoiesis with sustained lack of B1 cells and clinical aggravation of disease. Int. Immunol. 7:1879-1884. [PubMed] [Google Scholar]
- 13.Honarvar, N., U. E. Schaible, C. Galanos, R. Wallich, and M. M. Simon. 1994. A 14,000 MW lipoprotein and a glycolipid-like structure of Borrelia burgdorferi induce proliferation and immunoglobulin production in mouse B cells at high frequencies. Immunology 82:389-396. [PMC free article] [PubMed] [Google Scholar]
- 14.Hosmalin, A., M. Andrieu, E. Loing, J. F. Desoutter, D. Hanau, H. Gras-Masse, A. Dautry-Varsat, and J. G. Guillet. 2001. Lipopeptide presentation pathway in dendritic cells. Immunol. Lett. 79:97-100. [DOI] [PubMed] [Google Scholar]
- 15.Hovav, A. H., J. Mullerad, L. Davidovitch, Y. Fishman, F. Bigi, A. Cataldi, and H. Bercovier. 2003. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect. Immun. 71:3146-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, R. B., S. Kohl, and W. G. Bessler. 1983. Polyclonal activation of B-lymphocytes in vivo by Salmonella typhimurium lipoprotein. Infect. Immun. 39:1481-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleine, B., R. Sprenger, C. Martinez-Alonso, and W. G. Bessler. 1987. Polyclonal B-cell activation by a synthetic analogue of bacterial lipoprotein is functionally different from activation by bacterial lipopolysaccharide. Immunology 61:29-34. [PMC free article] [PubMed] [Google Scholar]
- 18.Kouskoff, V., S. Famiglietti, G. Lacaud, P. Lang, J. E. Rider, B. K. Kay, J. C. Cambier, and D. Nemazee. 1998. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J. Exp. Med. 188:1453-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 20.Lopez, M., L. M. Sly, Y. Luu, D. Young, H. Cooper, and N. E. Reiner. 2003. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J. Immunol. 170:2409-2416. [DOI] [PubMed] [Google Scholar]
- 21.Melchers, F., V. Braun, and C. Galanos. 1975. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J. Exp. Med. 142:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael, A., J. J. Hackett, M. Bennett, V. Kumar, and D. Yuan. 1989. Regulation of B lymphocytes by natural killer cells. Role of IFN-γ. J. Immunol. 142:1095-1101. [PubMed] [Google Scholar]
- 23.Montes, C. L., E. Zuniga, P. Minoprio, E. Vottero-Cima, and A. Gruppi. 1999. A Trypanosoma cruzi alkaline antigen induces polyclonal B-cell activation of normal murine spleen cells by T-cell-independent, BCR-directed stimulation. Scand. J. Immunol. 50:159-166. [DOI] [PubMed] [Google Scholar]
- 24.Reina-San-Martin, B., A. Cosson, and P. Minoprio. 2000. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol. Today 16:62-67. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, J. H., M. C. Case, and C. G. Brooks. 1992. Palmitic acid conjugation of a protein antigen enhances major histocompatibility complex class II-restricted presentation to T cells. Immunology 76:593-598. [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks, D. L., P. A. Scott, R. Asofsky, and F. A. Sher. 1984. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J. Immunol. 132:2072-2077. [PubMed] [Google Scholar]
- 27.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 29.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 30.Yoder, A., X. Wang, Y. Ma, M. T. Philipp, M. Heilbrun, J. H. Weis, C. J. Kirschning, R. M. Wooten, and J. J. Weis. 2003. Tripalmitoyl-S-glyceryl-cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect. Immun. 71:3894-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, D., C. Y. Koh, and J. A. Wilder. 1994. Interactions between B lymphocytes and NK cells. FASEB J. 8:1012-1018. [DOI] [PubMed] [Google Scholar]