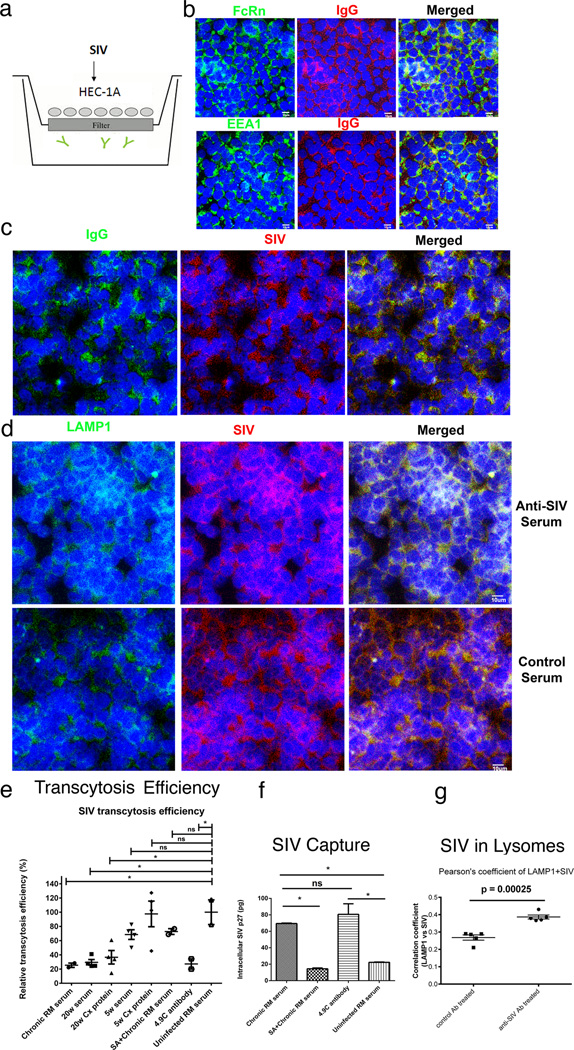
FIGURE 8.
Transcytosis, virus capture and intracellular neutralization (a). HEC 1-A-FcRn+ epithelial cell line and transwell system to assay SIV-capture, transcytosis and intracellular neutralization. SIV is added to the upper well. IgG antibodies (depicted as  ) are in the lower well (b) IgG and the FcRn co-localize in the EEA1+ early endosomal pathway. (c, d) SIV is captured in the FcRn transcytosis pathway and diverted to Lamp1+ lysosomes by anti-SIV sera but not control sera. (e, f) Quantification. Transcytosis of SIV in the transwell system is significantly decreased by antibodies from a SIV-infected rhesus macaque (RM); and sera and a cervical tissue extract (Cx Protein) from an animal at 20 weeks post vaccination and the rhesus monoclonal antibody 4.9C, which reacts identically in WBs to the cervical tissue antibodies to oligomeric gp41. Sera and cervical tissue extract (Cx protein) at 5 weeks post vaccination, and sera from uninfected RM do not significantly decrease transcytosis. The FcRn-translocation pathway was required for capture and transcytosis, since Staphylococcal protein A (SA), which blocks the interaction between FcRn and IgG, (20–21), diminished viral capture and inhibitory effect on viral transcytosis by SIV-infected RM serum. (g). Co-localization of SIV with LAMP1+ lysosomes in HEC 1-A cells treated with anti-SIV serum but not control serum from an uninfected animal. Extent of co-localization expressed as Pearson correlation coefficients. The small p values reflect the small group standard deviation relative to the magnitude of the difference between groups.
) are in the lower well (b) IgG and the FcRn co-localize in the EEA1+ early endosomal pathway. (c, d) SIV is captured in the FcRn transcytosis pathway and diverted to Lamp1+ lysosomes by anti-SIV sera but not control sera. (e, f) Quantification. Transcytosis of SIV in the transwell system is significantly decreased by antibodies from a SIV-infected rhesus macaque (RM); and sera and a cervical tissue extract (Cx Protein) from an animal at 20 weeks post vaccination and the rhesus monoclonal antibody 4.9C, which reacts identically in WBs to the cervical tissue antibodies to oligomeric gp41. Sera and cervical tissue extract (Cx protein) at 5 weeks post vaccination, and sera from uninfected RM do not significantly decrease transcytosis. The FcRn-translocation pathway was required for capture and transcytosis, since Staphylococcal protein A (SA), which blocks the interaction between FcRn and IgG, (20–21), diminished viral capture and inhibitory effect on viral transcytosis by SIV-infected RM serum. (g). Co-localization of SIV with LAMP1+ lysosomes in HEC 1-A cells treated with anti-SIV serum but not control serum from an uninfected animal. Extent of co-localization expressed as Pearson correlation coefficients. The small p values reflect the small group standard deviation relative to the magnitude of the difference between groups.