Figure 3.
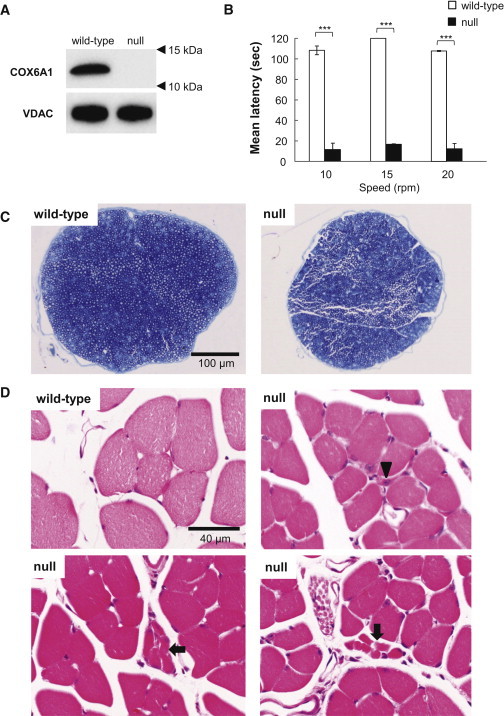
Characterization of Cox6a1 Knockout Mice
(A) Immunoblot of COX6A1 and voltage-dependent anion channel (VDAC) as control in a wild-type and Cox6a1 knockout null mice. Mice aged 7–8 weeks, three (one male and two female) knockout and three (two male and one female) wild-type were anesthetized and perfused with 10 mM PBS. Mitochondria fractions were obtained from liver tissue and applied to immunoblot. 20 μg of protein was loaded into a SDS-PAGE and transferred to the polyvinylidene difluoride membrane. The primary antibody for COX6A1 (mouse monoclonal, ab110265; abcam) is diluted 1:1,000 and secondary antibody for anti-COX6A1 (anti-mouse IgG, HRP-conjugated, 315-035-003; Jackson ImmunoResearch) is diluted 1:10,000. All blotting is carried out in 5% skim-milk/TBS solutions at room temperature for 1 hr.
(B) Motor coordination and balance was assessed as the latency to fall in the rota-rod. Mice aged 7–8 weeks, four (two male and two female) knockout and four (two male and two female) wild-type were used. Each mouse underwent the same 4 day procedure on a rota-rod (MK-660A; Muromachi Kikai). The first 3 days were used to train the mice (four sessions of 60 s each, walking at 20 rpm). The test sessions were run on the last day. The mice performed two series of three trials (10, 15, and 20 rpm) at each speed, with a 10 min rest period between trials. The latency to fall was recorded with a cut-off at 120 s. The difference between the wild-type and knockout null mice means were tested using t test. ∗∗∗p < 0.001. The error bars represent the standard deviation.
(C and D) Histological examinations by toluidine blue staining sections of mice sciatic nerves at lower magnification (C) and hematoxylin-eosin staining sections of mice lower limb muscles (D). Arrow indicates a smaller number of fibers are involved in small group atrophy and arrowhead indicates small angular fibers despite the limited numbers. Mice aged 7–8 weeks, four (two male and two female) knockout and four (two male and two female) wild-type were anesthetized and perfused with 10 mM PBS, followed by a fixative of 4% paraformaldehyde (for leg muscle) or 2.5% glutaraldehyde (for sciatic nerve) in 0.1 M phosphate buffer. Sciatic nerve specimens were fixed in 2.5% glutaraldehyde in phosphate buffer for 2 hr at room temperature. After postfixation with 1% OsO4, the tissues were embedded in epoxy resin. Tissue blocks were sectioned at 1 mm thickness and stained with toluidine blue for light microscopic examination. For the histological analysis of leg muscle, mice tissues were postfixed in 4% paraformaldehyde for 48 hr at 4°C. The muscle tissues were dissected out and then incubated overnight in 10% sucrose in phosphate buffer. After snap freezing with CO2 gas, tissue blocks were sectioned at 20 μm thickness in a cryostat and stained with hematoxylin and eosin.
