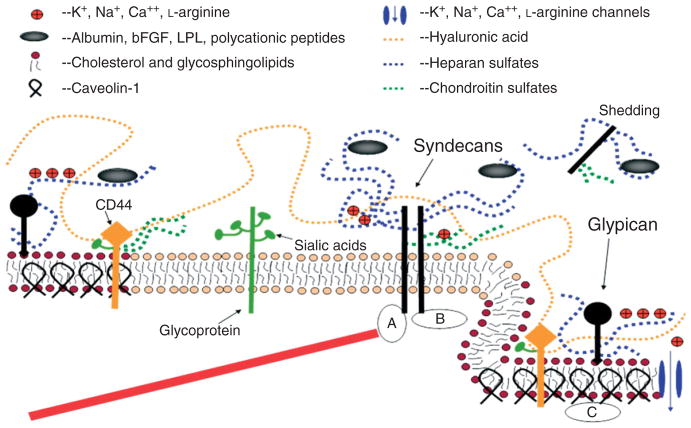
FIGURE 3.
A conceptual and simplified view for proteoglycans and glycosaminoglycans (GAGs) of the endothelial surface glycocalyx (ESG). Caveolin-1 associates with regions high in cholesterol and sphingolipids in the EC membrane (darker circles, left), and forms cave-like structures, caveolae (right). Glypicans, along with their HS chains (blue dotted lines) localize in these regions. Transmembrane syndecans are shown to cluster in the outer edge of caveolae. Besides HS, syndecans also contain CS, lower down the core protein (green dotted lines). A glycoprotein with its short oligosaccharide branched chains and their associated SA ‘caps’ are displayed in the middle part of the figure (green). HA is a very long GAG (orange dotted line), which weaves into the glycocalyx and binds with CD44. Transmembrane CD44 can have CS, HS and oligosaccharides attached to it, and localizes in caveolae. Plasma proteins (gray), along with cations and cationic amino acids (red circles) are known to associate with GAGs. (a) The cytoplasmic domains of syndecans can associate with linker molecules which connect them to cytoskeletal elements (red line). (b) Oligomerization of syndecans helps them make direct associations with intracellular signaling effectors. (c) A series of molecules involved in eNOS signaling localize in caveolae. (Reprinted with permission from Ref 18. Copyright 2006 Blackwell Publishing Ltd)