Fig. 13.
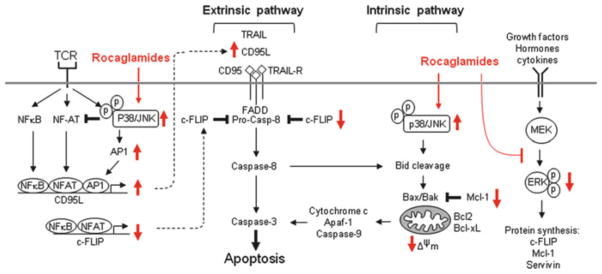
Apoptosis pathways affected by rocaglamides. Apoptotic cell death is regulated by two main pathways: extrinsic (receptor-mediated) and intrinsic (mitochondria-mediated) pathways. The extrinsic pathway involves ligation of death receptors (e.g. CD95 and TRAIL-R) with their ligands (e.g. CD95L and TRAIL) resulting in a sequential activation of caspase-8 and -3, which cleave target proteins leading to apoptosis. This pathway is negatively regulated by the anti-apoptotic protein c-FLIP. Intrinsic stimuli (e.g. anticancer drugs) directly or indirectly activate the mitochondrial pathway by inducing release of cytochrome c and activation of caspase-9. Caspase-9, in turn, activates caspase-3. This death pathway is largely controlled by the pro-apoptotic (e.g. Bax and Bak) and anti-apoptotic (e.g. Mcl-1, Bcl-2 and Bcl-xL) proteins. Activated caspase-8 may induce cleavage of Bid, which induces translocation of Bax and/or Bak to the mitochondrial membrane and amplifies the mitochondrial apoptosis pathway. Bid cleavage can be also induced by activated p38 and JNK. Several rocaglamides can activate p38 and JNK leading to Bid cleavage. Rocaglamides can also directly inhibit protein synthesis by interfering with eIF4A (see Fig. 9) or indirectly through inhibition of the MEK-ERK-eIF4E pathway. Protein synthesis inhibition will lead to down-regulation of short-lived anti-apoptotic proteins such as c-FLIP and Mcl-1. Furthermore, rocaglamides may further increase T-cell-receptor (TCR)-mediated activation of p38 and JNK leading to down-regulation of NF-AT activity and up-regulation of AP-1 activity. This event results in up-regulation of CD95L promoter activity and suppression of c-FLIP promoter activity leading to enhancing activation-induced-cell-death
