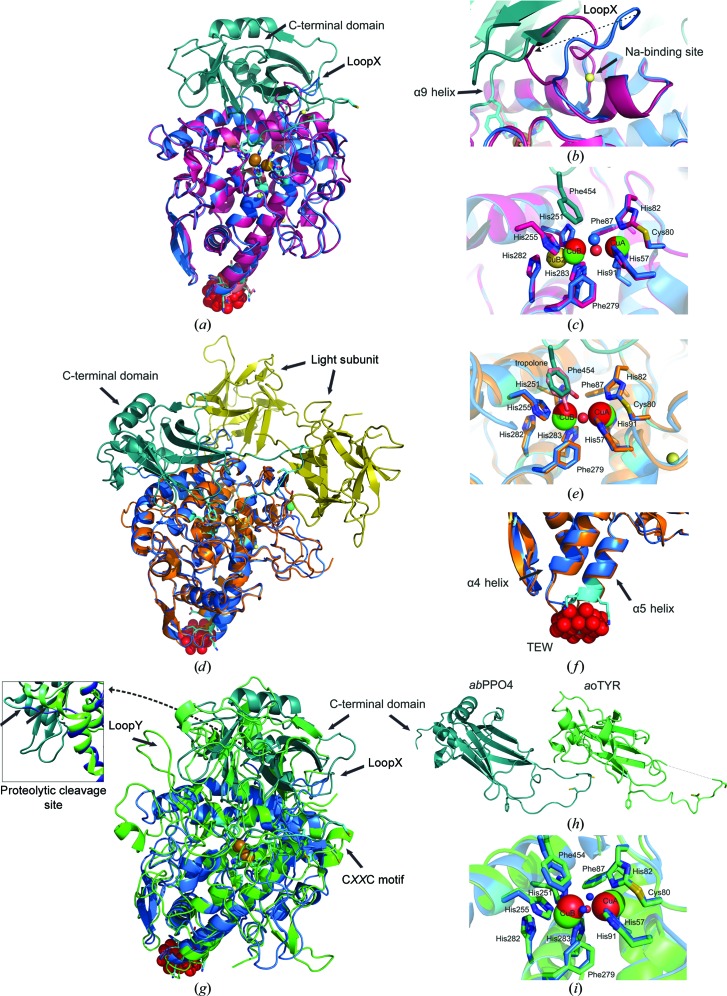
Figure 4.
Superimpositions. Colour code: L-TYR main core, blue; L-TYR C-terminal domain, turquoise; A-TYR main core, purple; aoTYR main core, green; aoTYR C-terminal domain, lime green; abPPO3 main core, orange; abPPO3 light subunit, yellow; L-TYR abPPO4 copper ions, green; respective superimposed copper ions, red; sodium ions, yellow; water molecules, blue; tropolone, pink; POM oxygen spheres, red. (a) Superimposition of L-TYR abPPO4 with A-TYR abPPO4. (b) Magnified region of loopX (Asn236–Ser246) which is pushed sideways by the attachment of the C-terminal domain. The dashed arrow indicates the motion of loopX owing to the removal of the C-terminal domain. A sodium ion occupying the respective metal-binding site of loopX in L-TYR is thereby lost. (c) Magnified superimposition of the respective active sites (A-TYR and L-TYR). (d) Superimposition of L-TYR abPPO4 with A-TYR abPPO3. (e) Magnified superimposition of the respective active sites (L-TYR abPPO4 and A-TYR abPPO3) depicting a similar location of the placeholder Phe454 and the inhibitor tropolone. (f) Superimposed POM-binding region of L-TYR abPPO4 and abPPO3 showing that no conformational change is induced owing to POM binding. (g) Superimposition of L-TYR abPPO4 with aoTYR. The two loops putatively pushed sideways owing to the attachment of the C-terminal domain of aoTYR are indicated as loopX and loopY, respectively. The insert shows the superimposed proteolytic cleavage site located on the back side in the respective perspective of the main view. In contrast to an α-helical location in aoTYR, the site is located on a loop in abPPO4. (h) Parallel arranged C-terminal subunits of L-TYR abPPO4 (left) and aoTYR (right) for a proper comparison. The dashed line indicates missing residues. (i) Magnified superimposition of the respective active sites (L-TYR abPPO4 and aoTYR).