Figure 6.
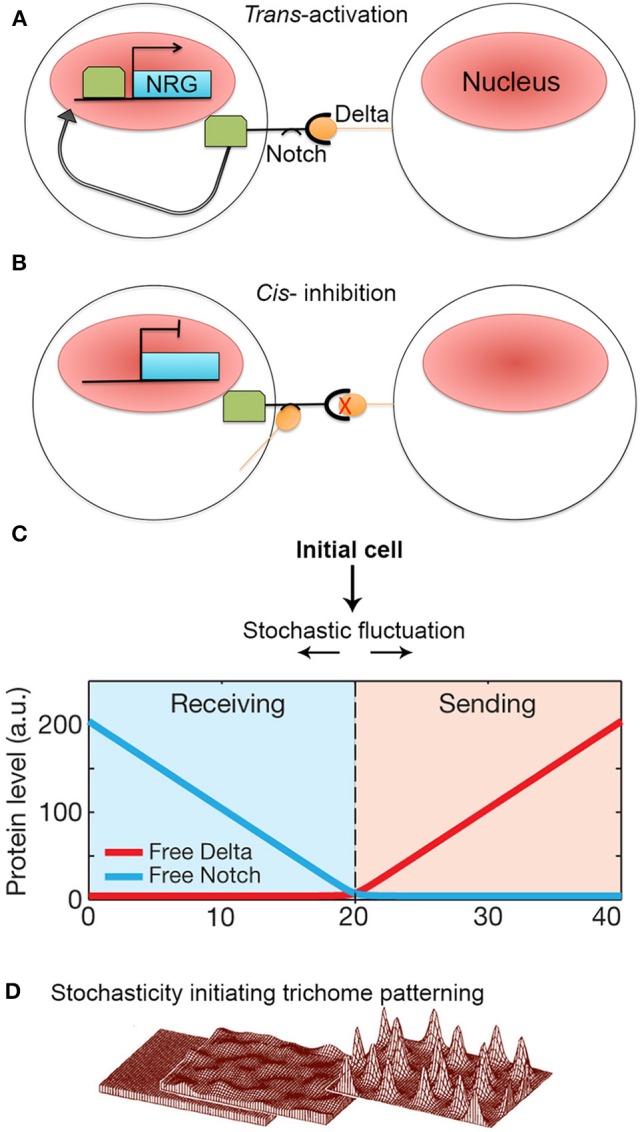
Stochasticity initiates lateral inhibition-based patterning. (A) A simplified model of the Notch-Delta pathway. When transmembrane receptor Notch on one cell interacts with ligand Delta on the neighboring cell, Notch is cleaved and intracellular domain subsequently moves to the nucleus to trans-activate Notch-responsive genes (NRG). (B) However, if Delta interacts with Notch in the same cell, Notch is inhibited and unable to activate NRGs. Additionally, Notch becomes less responsive to trans-Delta. (C) When Notch and Delta are present in the same cell, they mutually inactivate one another. This creates a “switching” environment, where small concentration imbalances between Notch and Delta due to stochastic fluctuations will either cause a cell to transition to a sending state (high delta, low notch) or a receiving state (low delta, high notch) thus activating the patterning process. Reprinted with permission from MacMillan Publishers Ltd: Sprinzak et al. (2010). (D) Computational models of de novo trichome patterning based on Meinhardt's model, which suggests that trichome patterning is initiated via small stochastic changes in activator and inhibitor concentrations in equivalent cells (left to middle). Regulatory positive feedback loops to amplify small concentration differences. In addition inhibitor factors prevent surrounding cells from differentiating into trichomes producing a spaced pattern (middle to right). Reprinted by permission from MacMillan Publishers Ltd: Hülskamp (2004).
