Abstract
Marginal structural models (MSMs) and inverse probability weighting can be used to estimate risk in a cohort of active workers if there is a time-varying confounder (e.g., health status) affected by prior exposure—a feature of the healthy worker survivor effect. We applied Cox MSMs in a study of incident ischemic heart disease and exposure to particulate matter with aerodynamic diameter of 2.5 μm or less (PM2.5) in a cohort of 12,949 actively employed aluminum workers in the United States. The cohort was stratified by work process into workers in smelting facilities, herein referred to as “smelters” and workers in fabrication facilities, herein referred to as “fabricators.” The outcome was assessed by using medical claims data from 1998 to 2012. A composite risk score based on insurance claims was treated as a time-varying measure of health status. Binary PM2.5 exposure was defined by the 10th-percentile cutoff for each work process. Health status was associated with past exposure and predicted the outcome and subsequent exposure in smelters but not in fabricators. In smelters, the Cox MSM hazard ratio comparing those always exposed above the cutoff with those always exposed below the cutoff was 1.98 (95% confidence interval: 1.18, 3.32). In fabricators, the hazard ratio from a traditional Cox model was 1.34 (95% confidence interval: 0.98, 1.83). Results suggest that occupational PM2.5 exposure increases the risk of incident ischemic heart disease in workers in both aluminum smelting and fabrication facilities.
Keywords: epidemiologic methods, healthy worker effect, occupational epidemiology
Exposure to particulate matter with aerodynamic diameter of 2.5 μm or less (PM2.5) in air pollution has been associated with cardiovascular morbidity and death (1–6). Most of the literature on particulate matter (PM) and cardiovascular disease deals primarily with ambient air pollution and active or secondhand smoking (7), with more limited evidence for occupational exposures from industrial sources (8–11). Although heart disease is not often the primary outcome of interest in workplace-based studies (12), occupational environments can provide a range of exposures and sources that complement the existing research on PM and cardiovascular health risks. Occupational studies, however, are often limited by the unavailability of data on many potential confounders (11), as well as by the ubiquitous healthy worker effect (13).
We recently described results from a prospective cohort in the US aluminum industry in which elevated hazard ratios for incident ischemic heart disease (IHD) were associated with higher exposure to PM2.5 (14). Workers in the aluminum industry are widely exposed to PM during several stages in the manufacturing process (15), with high exposures particularly in smelting facilities. A statistically significant dose response was seen in workers in fabrication facilities (herein referred to as “fabricators”), but results in workers in smelting facilities (herein referred to as “smelters”), in whom exposures were almost an order of magnitude higher, were attenuated (14). Differences in exposure composition and exposure misclassification, as well as the healthy worker survivor effect (HWSE), a part of healthy worker effect, were cited as potential reasons for the differences in exposure response between the 2 subcohorts defined by work process.
The PM to which smelters are exposed is composed of a mixture of dusts and fumes produced during smelting operations, and personal exposures often reach high levels given the proximity of workers to sources of combustion. Smelting dust is composed of inorganic materials such as fluorides, metals, and coal tar volatiles, which have been associated with adverse heart disease endpoints in this and other industries (16–18). By contrast, PM in fabrication facilities is composed primarily of water-based soluble and synthetic metalworking fluids, as well as metal dust (19). Soluble fluids contain mineral oils, and oil-based straight (as opposed to soluble or synthetic) metalworking fluids have been associated with IHD risk in other manufacturing sectors (20–22).
The HWSE is a bias caused by a time-varying confounder that is affected by past exposure, as illustrated in the directed acyclic graph in Figure 1. In the graph for this study, past exposure (Et, high PM2.5) is harmful to health (the time-varying confounder Lt+1, a comprehensive health risk score) and, subsequently, less healthy individuals are less likely to be exposed (Et+1, high PM2.5). Marginal structural models (MSMs) with inverse probability weighting better address this time-varying confounding and can provide unbiased causal effect estimates under certain assumptions (23–26). This method was recently implemented in a prospective study of work-related asthma to address the HWSE (27). In the present study, we address the HWSE in the aluminum industry study mentioned above by examining the data structure and implementing MSMs in 2 subcohorts of aluminum workers (in smelting and fabrication facilities) to assess the relationship of PM2.5 with the risk of IHD.
Figure 1.
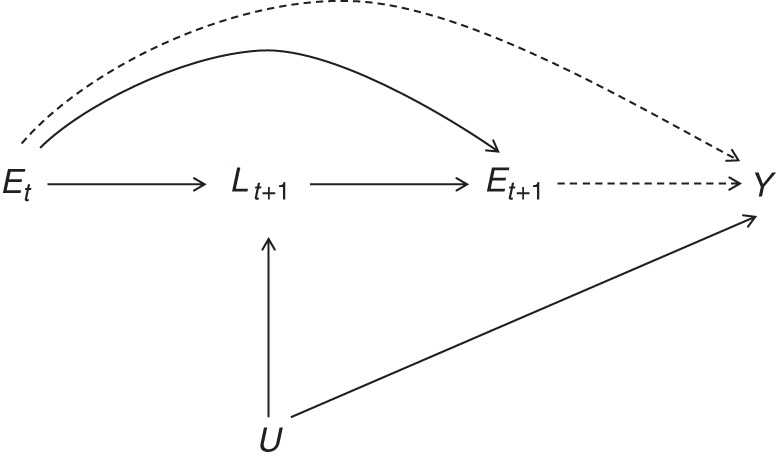
Directed acyclic graph representation of the healthy worker survivor effect in a study population of active US aluminum industry workers, 1998–2012. An indication for poor health status (L), which is also associated with the outcome (Y), is affected by previous exposure (Et) and, in turn, reduces the probability of subsequent exposure (Et+1). U represents a common cause of intermittent health status L, and the outcome Y and subscripts indicate time points in a longitudinal study.
METHODS
Study population and outcome assessment
We conducted our analysis using data from hourly workers at 11 US plants of the same aluminum company. Workers had to be enrolled in the primary insurance plan and employed for at least 2 years during follow-up to be eligible. To exclude prevalent cases, we required a 2-year washout period without any IHD claims. Follow-up began on January 1, 1998, (after the application of the 2-year inclusion criterion) for most plants and on January 1, 2003, for the 2 facilities acquired by the company at a later date. Subjects were enrolled either at the start of follow-up or on their dates of hire (plus 2 years), whichever came first.
Incident IHD cases were identified from health insurance claims through 2012 or until the date of active employment termination (whichever occurred first). IHD cases were defined as subjects with insurance claims for relevant procedures (i.e., revascularization, angioplasty, bypass), hospitalization for 2 or more days, or face-to-face visits with International Classification of Diseases, Ninth Revision, admission codes for IHD (codes 410–414) or death from IHD (identified by International Classification of Diseases, Ninth Revision, codes 410–414 or International Classification of Diseases, Tenth Revision, codes I20–I25) while actively employed and without any previous incident IHD event. Deaths were identified from the National Death Index (National Center for Health Statistics, Hyattsville, Maryland) through 2011.
Covariate information
Information was available on age, sex, race, and job grade through employment records. Data on smoking status, height, and weight were collected at occupational health clinics located at each of the facilities, and availability varied by facility. We used multiple imputation for the missing data on smoking and body mass index (BMI) (weight (kg)/height (m)2). Information was also available on dates of diagnosis of hypertension, diabetes, and dyslipidemia during follow-up. In addition to these medical conditions, we also had access to a time-varying health risk score, derived using a third-party algorithm (Sightlines DxCG Risk Solutions software, Verisk Analytics, Inc., Jersey City, New Jersey) to predict future health expenditures for insurance purposes. The scores were based on medical and pharmacy data using Current Procedural Terminology codes, International Classification of Diseases codes, use of health care services, and risk-adjustment algorithms. They were comparable to those used by the Centers for Medicare and Medicaid Services and other insurance bodies (28, 29), and yearly values were available. The risk score has been found to be associated with a variety of health outcomes, including diabetes complications, acute injury, and death (29–31); this variable was the proxy for overall health status in our analysis.
Exposure assessment
Average annual PM2.5 concentrations (in mg/m3) were assigned to distinct exposure groups within each plant to create a job exposure matrix. The estimates were based on more than 8,000 industrial hygiene samples collected over 25 years by the company, as well as measurements collected by our research team in 2010–2011. The exposure assessment has been described in detail elsewhere (25). Exposure estimates for each job-year were classified by confidence level. “High confidence” was assigned if estimates were based on direct measurements of total PM rather than extrapolation. As in previous analysis of these data (14), we restricted person-time to years with a high-confidence PM2.5 exposure value, which accounted for approximately 80% of all observations. A dichotomous exposure variable was defined for each subgroup using the 10th percentile of the distribution of PM2.5 across person-time. We also considered a categorical exposure with values below the 10th percentile as the reference group and 4 higher categories determined by quartiles of the remaining distribution.
Statistical analysis
Traditional analysis
All analyses were stratified by work process (smelting or fabrication). Standard Cox proportional hazards regression models were fitted to estimate hazard ratios for the PM2.5 exposure and incident IHD. Attained age was used as the time scale, and models were stratified so that baseline hazards were allowed to vary by decade of age. Models also included covariates for sex, race (white or nonwhite), job grade (dichotomous variable, defined as above or below the median for each facility), smoking status (current, ever, or never), plant, and BMI value. Risk score was entered in the models as a continuous variable after it was recoded into deciles. Approximately 8% of the observations were omitted because of a lack of risk score data.
Multiple imputation using the MI procedure in SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina) was used to impute missing data for smoking (60% missing) and BMI (30% missing). Given the sources of the smoking and BMI data, it was plausible to assume the data were missing at random after accounting for case status and the variables included in the main analytical models. We first imputed BMI values using the expectation-maximization algorithm (32). Smoking status was subsequently imputed using the logistic regression method.
To examine whether traditional models sufficed in each subcohort analysis, we examined pathways (Figure 1) by estimating associations between past exposure and subsequent health status (i.e., risk score) and between health status and subsequent exposure. If the latter association is not present, there is no need to adjust for health status; if the former association is not present, adjustment using a conventional Cox model is appropriate. If both associations are present, then the MSM is a more appropriate approach. To examine whether exposure in the past year was predictive of risk score, we fitted linear regression models for decile of risk score, adjusted for the same covariates as outcome models (i.e., lagged risk score and previous diagnoses of diabetes, hypertension, and dyslipidemia).
MSMs and inverse probability weighting
For the MSMs, pooled logistic models for the annual exposure were fitted to determine the inverse probability weights. The models included covariates for age, sex, race, smoking status, BMI, job grade, and plant, as well as exposure status (as a dichotomous variable) in the previous year. Risk score estimation was based on past data and, thus, chronologically precedes exposure in each person-year (Figure 1); it was entered in the model without lagging. Estimation of inverse probability weights has been described in detail elsewhere (24, 25). Briefly, the weights for each subject at a given time point are proportional to the inverse of the probability that each subject had his or her own actual exposure history at a given time. These probabilities were estimated as the product of the probability of a subject receiving his or her own exposure in each year using predicted values from the logistic models described above, resulting in subject-specific, time-dependent weights (per person-year). Stabilized weights were estimated to minimize variability and extreme weight values (23). The same process was repeated for the categorical exposure variable with the use of multinomial logistic models.
Cox models with robust variance estimation were then fitted using the pseudopopulation created by the inverse probability weights in which the pathway of exposure to outcome is no longer confounded by intermediate health status (i.e., the arrow from Lt+1 to Et+1 in Figure 1 is no longer present). As in the standard models, attained age was the time scale, and the rest of the covariates listed above (with the exception of risk score) were also included in the models.
Primary analyses included imputed smoking status and BMI data. A sensitivity analysis was also conducted using only available smoking data with a categorical smoking variable coded as current, ever, never, or missing.
Inverse probability of censoring weights
Censoring occurred at employment termination prior to the administrative end of follow-up, and was due to retirement, layoff, transition to salary (no longer working as an hourly worker), or voluntary quitting. Opting out of the primary health plan also resulted in censoring because participation in the plan was required for ascertainment of the outcome. The possibility that censoring was related to both the health outcome and exposure (i.e., informative censoring) was addressed through the use of inverse probability of censoring weights. For this purpose, we considered terminations prior to the age of 55 years, without evidence of a layoff, transition to salary, or change in health claim eligibility as less likely to be related to normal retirement. Censoring weights were calculated using logistic models for the probability of remaining uncensored through each year on the basis of exposure, in addition to the same predictors as those included to estimate the exposure weights. Stabilized weights were used. Cox models were then fitted to the pseudopopulation that resulted from applying both exposure and censoring weights. We also considered less restrictive definitions, treating all employment terminations of workers under the age of 55 years as censored, and finally, all terminations, regardless of worker age, as censored.
RESULTS
Demographic characteristics for the cohort, stratified into 2 subgroups, are summarized in Table 1. The final sample consisted of 5,555 smelters and 7,394 fabricators. The cohort was predominantly composed of white men, especially in the smelting facilities. Overall, the characteristics of workers in the 2 types of facility were very similar at baseline, with fabricators appearing slightly less healthy, as indicated by a higher risk score. The crude IHD incidence rate over the duration of follow-up was also slightly higher in fabricators (7.1%) than in smelters (6.2%). Exposures were considerably higher in smelting facilities, with a mean of 2.17 mg/m3 (median, 1.96 mg/m3) versus a mean of 0.33 mg/m3 (median, 0.20 mg/m3) in fabrication facilities. The 10th percentiles were 0.26 mg/m3 in smelting facilities and 0.07 mg/m3 in fabrication facilities.
Table 1.
Demographic Characteristics of a Cohort of Actively Employed US Aluminum Workers at the Start of Follow-up, Stratified by Facility Type, 1998–2012
| Characteristic | Smelting Facilities |
Fabrication Facilities |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | Median (Range) | No. | % | Mean (SD) | Median (Range) | |
| No. of workers | 5,555 | 7,394 | ||||||
| Person-years | 35,554 | 48,119 | ||||||
| Length of follow-up, years | 6.73 (4.35) | 6.93 (4.60) | ||||||
| No. of IHD cases | 347 | 6.2 | 525 | 7.1 | ||||
| Year of hire | 1989 (1944–2010) | 1989 (1949–2010) | ||||||
| Male sex | 5,250 | 94.5 | 5,962 | 80.6 | ||||
| White race | 4,778 | 86.0 | 6,064 | 82.0 | ||||
| Age, years | 43.19 (10.19) | 44.10 (9.93) | ||||||
| Body mass indexa | 29.29 (5.11) | 29.60 (5.57) | ||||||
| Smoking statusa | ||||||||
| Current | 27.2 | 27.4 | ||||||
| Ever | 34.6 | 30.3 | ||||||
| Diabetes | 313 | 5.6 | 429 | 5.8 | ||||
| Hypertension | 1,025 | 18.5 | 1,229 | 16.6 | ||||
| Dyslipidemia | 968 | 17.4 | 1,234 | 16.7 | ||||
| Risk score | 0.85 (0.15–54.98) | 0.90 (0.15–51.07) | ||||||
Abbreviations: IHD, ischemic heart disease; SD, standard deviation.
a Body mass index (weight (kg)/height (m)2) data were available for approximately 70% of the study population, and smoking status data were available for 40%. The values presented here are based only on the available data.
Exposure was a statistically significant predictor in linear regression models of risk score in smelters, with a higher risk score associated with exposure. By contrast, past exposure did not predict risk score in fabricators (Table 2). Lagged risk score, age, and diagnosis of diabetes, hypertension, or dyslipidemia were strong predictors of the risk score variable in both smelters and fabricators.
Table 2.
Associations Between Time-Varying Exposure to PM2.5 and Health Risk Score Variables in a Cohort of Actively Employed US Aluminum Workers, 1998–2012a
| Facility Type | Change in Risk Score Associated With Past Exposureb |
Probability of Being Exposed Associated With a 1-Decile Increase in Risk Scorec |
||
|---|---|---|---|---|
| Change | 95% CI | OR | 95% CI | |
| Smelting | 0.07 | 0.00, 0.13 | 0.94 | 0.91, 0.96 |
| Fabrication | 0.03 | −0.03, 0.08 | 0.96 | 0.94, 0.98 |
Abbreviations: CI, confidence interval; OR, odds ratio; PM2.5, particulate matter with aerodynamic diameter of 2.5 μm or less.
a Exposure defined using the 10th percentile as cutoff.
b Linear regression model controlling for risk score in the previous year; age; sex; race; smoking status; body mass index (weight (kg)/height (m)2); job grade; plant; and diagnosis of diabetes, hypertension, or dyslipidemia.
c Logistic regression model controlling for exposure status in the previous year, age, sex, race, smoking status, body mass index, job grade, and plant.
On the basis of logistic regression, risk score was a statistically significant predictor of subsequent exposure in both smelters and fabricators, with odds ratios less than 1.00. Exposure in the previous year, however, was the strongest predictor for subsequent exposure in both strata. Finally, risk score was also a strong predictor of incident IHD, with hazard ratios of 2.24 (95% confidence interval (CI): 2.02, 2.48) and 1.99 (95% CI: 1.84, 2.16) associated with each 1-decile increase in risk score for smelters and fabricators, respectively. The means of the computed inverse probability of exposure weights were 1.00 (standard deviation, 0.09; range, 0.29–3.76) in smelters and 1.00 (standard deviation, 0.10; range, 0.27–4.27) in fabricators.
Hazard ratios from Cox MSMs were higher than traditional Cox hazard ratios in smelters, but not in fabricators (Figure 2), with a hazard ratio in smelters of 1.98 (95% CI: 1.18, 3.32) comparing those always exposed above the cutoff versus those always exposed below the cutoff. The hazard ratio from a traditional Cox model for fabricators, unadjusted for risk score, was 1.24 (95% CI: 0.92, 1.68). A traditional Cox model adjusting for risk score yielded a hazard ratio of 1.34 (95% CI: 0.98, 1.83). Results from a sensitivity analysis using only available data on smoking (not imputed values) were not substantially different. Cox MSMs yielded higher hazard ratios than traditional models in smelters when the categorical exposure variable was considered. The highest hazard ratios were observed for the first 2 exposure categories compared with the reference category, whereas hazard ratios for the 2 higher exposure categories were somewhat lower (Table 3).
Figure 2.
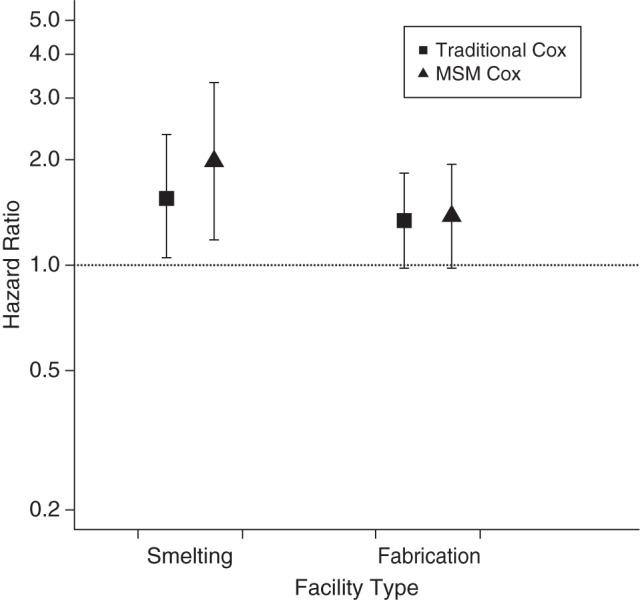
Hazard ratios from both traditional Cox models and Cox marginal structural models (MSMs) for the risk of ischemic heart disease comparing those always exposed above the 10th-percentile exposure distribution cutoff of particulate matter with aerodynamic diameter of 2.5 μm or less with those always exposed below the cutoff in smelting and fabrication facilities in a cohort of active US aluminum industry workers, 1998–2012. Bars, 95% confidence intervals.
Table 3.
Hazard Ratiosa Associated With Categorical Exposures to PM2.5 in a Cohort of Actively Employed US Aluminum Workers, Stratified by Facility Type, 1998–2012
| Exposure Category, mg/m3, by Facility Type | Traditional Cox Modelb |
Cox MSM/IPWc |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Smelting facilities | ||||
| <0.260 | 1.00 | Referent | 1.00 | Referent |
| 0.260–1.469 | 1.51 | 0.98, 2.37 | 2.00 | 1.16, 3.45 |
| 1.470–1.959 | 1.73 | 1.06, 2.86 | 1.97 | 1.06, 3.67 |
| 1.960–2.589 | 1.53 | 0.97, 2.45 | 1.78 | 1.00, 3.18 |
| ≥2.590 | 1.53 | 0.97, 2.46 | 1.77 | 1.01, 3.11 |
| Fabrication facilities | ||||
| <0.07 | 1.00 | Referent | 1.00 | Referent |
| 0.07–0.139 | 1.34 | 0.96, 1.88 | 1.35 | 0.96, 1.88 |
| 0.140–0.219 | 1.29 | 0.90, 1.87 | 1.18 | 0.83, 1.69 |
| 0.220–0.369 | 1.29 | 0.89, 1.86 | 1.31 | 0.90, 1.89 |
| ≥0.370 | 1.31 | 0.92, 1.89 | 1.23 | 0.87, 1.75 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IPW, inverse probability weight; MSM, marginal structural model; PM2.5, particulate matter with aerodynamic diameter of 2.5 μm or less.
a Observations below the 10th percentile of exposure are the reference group.
b Cox models with attained age as time scale, including covariates for sex, race, smoking status, body mass index (weight (kg)/height (m)2), job grade, plant, and risk score. Baseline hazards based on decade of age.
c Cox models with attained age as time scale fitted in a pseudopopulation created by use of inverse probability weights, including covariates for sex, race, smoking status, body mass index, job grade, and plant. Baseline hazards based on decade of age.
Most subjects who left follow-up prior to the administrative end of the study did so because of termination of employment. To adjust for informative censoring, we also estimated the inverse probability of censoring weights. Odds ratios from logistic models for the probability of remaining uncensored indicated that more highly exposed individuals were more likely to remain uncensored. Risk score was also predictive of censoring; healthier people with lower risk scores were less likely to be censored. However, adjustment with censoring weights using the strictest definition of informative censoring did not significantly affect the hazard ratio estimates (Table 4).
Table 4.
Associations Between Binary Exposure to PM2.5 and Censoringa and Incident IHD Hazard Ratios for Binary Exposure Based on Cox Marginal Structural Models With and Without Censoring Weightsb in a Cohort of Actively Employed US Aluminum Workers Stratified by Facility Type, 1998–2012
| Definition of Censoring Used to Create Weights, by Facility Type | Probability of Remaining Uncensored Associated With Exposure to PM2.5 |
Risk of IHD Associated With Exposure to PM2.5 |
||
|---|---|---|---|---|
| OR | 95% CI | HR | 95% CI | |
| No censoring weights | ||||
| Smelting | 1.98 | 1.18, 3.32 | ||
| Fabrication | 1.38 | 0.98, 1.94 | ||
| All terminationsc | ||||
| Smelting | 1.07 | 0.95, 1.20 | 1.87 | 1.10, 3.18 |
| Fabrication | 1.05 | 0.94, 1.16 | 1.35 | 0.95, 1.91 |
| <55 Years of age at terminationd | ||||
| Smelting | 1.11 | 0.96, 1.28 | 1.96 | 1.17, 3.29 |
| Fabrication | 0.92 | 0.81, 1.05 | 1.36 | 0.97, 1.91 |
| <55 Years of age and not laid off, transferred to salary, or having lost claims eligibilitye | ||||
| Smelting | 1.25 | 1.05, 1.48 | 1.95 | 1.17, 3.27 |
| Fabrication | 1.09 | 0.92, 1.30 | 1.37 | 0.97, 1.93 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IHD, ischemic heart disease; OR, odds ratio; PM2.5, particulate matter with aerodynamic diameter of 2.5 μm or less.
a From logistic models for the probability of remaining uncensored, including covariates for sex, race, smoking status, body mass index (weight (kg)/height (m)2), job grade, plant, and risk score.
b Hazard ratio comparing those always exposed above with those always exposed below the 10th percentile of PM2.5 cutoff.
c A total of 2,641 workers in smelting facilities and 3,707 workers in fabrication facilities were considered censored.
d A total of 1,446 workers in smelting facilities and 2,210 workers in fabrication facilities were considered censored.
e A total of 956 workers in smelting facilities and 1,178 workers in fabrication facilities were considered censored.
DISCUSSION
We observed an increase in the risk of IHD when comparing workers exposed above versus below the 10th percentile of the PM2.5 exposure distribution in both smelting and fabrication facilities. Risk score, a time-varying confounder in both the smelter and fabricator subcohorts, was associated with prior exposure only in smelters. The hazard ratios were higher in Cox MSMs than in traditional Cox models in the smelter subcohort, where the pathway from exposure to outcome more closely resembles the relationships illustrated in Figure 1. This finding provides a partial explanation for the weaker association previously reported in smelting facilities than in fabrication facilities, which is the finding that motivated this study (14).
By contrast, PM2.5 exposure in fabrication facilities did not appear to affect intermediate health status, although there was still a suggestion of higher risk of incident IHD. The absence of an association between past exposure and the time-varying confounder suggests there is no arrow from Et to Lt+1 (Figure 1). Therefore, results from the traditional Cox models should be considered unbiased for the fabricator subcohort, provided the assumptions of no unmeasured confounding and correct model specification hold. Indeed, results from a traditional model and a MSM were not substantially different.
Overall, our results indicate that occupational exposure to PM2.5 in the aluminum industry is associated with higher risk of incident IHD. The relative risk was higher in smelters than in fabricators when comparing exposures above and below the 10th percentile of PM2.5 distribution. This difference is likely explained by the much higher exposure range in smelting facilities. We also considered a categorical exposure using multinomial logistic regression to estimate the inverse probability weights. All hazard ratios were elevated, and significantly so in the smelters. Results did not support a linear dose response, however, and associations in the highest categories were slightly lower than in the middle categories among smelters. The lack of trend may be partially explained by exposure misclassification and company screening programs, as described below.
Respirator use was not considered in the estimation of PM2.5 values. Respirators to protect against PM are more frequently used in the jobs with higher PM exposure, and this would likely lead to greater misclassification in the higher end of the exposure range, and more so in smelting facilities. The use of a simple binary measure of exposure is probably less likely to be affected by exposure misclassification, especially at the lower ends of the exposure distribution, than a categorical variable.
Furthermore, the company has a posthire job placement program for smelters, which is designed to place higher-risk individuals in less physically demanding and hazardous jobs (14). Differential health screening for jobs with respect to exposure may result in a “healthy hire” effect even within a single facility, if the more highly exposed individuals are more thoroughly screened and, therefore, at lower risk for heart disease. Both of these limitations could undermine an analysis of continuous exposure that assumes a linear dose response.
In this cohort, no workers were truly “unexposed”; even the 10th percentile of exposure was several orders of magnitude higher than the US Environmental Protection Agency (Washington, DC) environmental air quality standard of 15 µg/m3for annual average ambient PM2.5. Our choice of exposure cutoff for this internal comparison was based on the exposure distribution and limited by power considerations. Implementation of inverse probability of exposure weights is more straightforward with dichotomous exposures, though inferences about exposure-response relationships are more limited. The distributional assumptions required for applying inverse probability of treatment weights to continuous exposure metrics (23) were not met in this study; the PM2.5 data were highly skewed.
MSMs are 1 of several alternative g-methods that have been developed to address time- varying confounders affected by prior exposure (23, 24, 33–36). Assuming correct models (both for weights and main analysis), conditional exchangeability given the observed covariates (or no unmeasured confounding), and positivity, MSMs can provide consistent estimates of average causal effects of exposure (25). Positivity requires that the probability of receiving exposure is non-0 for all nonempty combinations of covariates. In studies in which follow-up extends beyond active employment, and active employment status is used as a proxy for a time-varying overall health variable, there is a positivity violation because those who are not actively employed are, by definition, unexposed. This, however, is not the case in occupational studies of active workers (20, 23). In the present study, the probability of receiving exposure is non-0 for all levels of the time-varying confounder (risk score), and MSMs can therefore be used, in addition to g-estimation of structural nested models and the parametric g-formula. MSMs provide the advantage of being less computationally intensive than structural nested models, and they allow the estimation of logistic or Cox model parameters, as in traditional survival analysis (24). Follow-up in this study was restricted to active employment because outcomes were based on medical claims available only for those still at work.
Previous studies that have attempted to control for the HWSE as a time-varying confounder affected by prior exposure have used proxies for intermittent health status, such as time off work (30, 37, 38). In this study, a time-varying risk score, a comprehensive health status variable designed to predict future health expenditures, was available. This risk score is a more direct measure of health status; it was found to be a strong predictor of the IHD outcome in this cohort study and has also been shown to predict a variety of health outcomes (28–31). Unlike most occupational cohort studies, the present study included information on many other potential confounders, although some missing data on cigarette smoking and BMI were a limitation.
Approximately half of the hourly workers in this study did not reach the administrative end of follow-up or develop the outcome. Retirement was the most likely reason for termination, and, therefore, the end of follow-up. Other causes included voluntary quitting, company-initiated termination, transition from hourly work to salary, or change in health claims eligibility (the latter 2 being exclusion criteria for this cohort). At certain time points during follow-up, layoffs and accelerated retirements also occurred at various levels across facilities because of downsizing and cuts in production brought on by economic recession (28). As described in the general terms suggested by Hernán et al. (39), censoring in this study is likely related to exposure both directly and indirectly through unmeasured economic and other industry-related factors (E → C and E ← U2 → C paths, Figure 3). This, as well as lack of detailed information on the reason for termination of employment at the individual level, made classifying terminations into uninformative versus informative censoring especially challenging.
Figure 3.
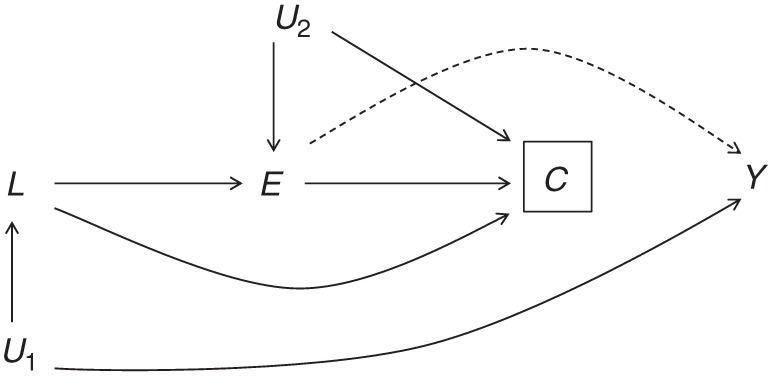
Directed acyclic graph representation of potentially informative censoring in a study population of active US aluminum industry workers, 1998–2012. Censoring (C) is connected to the outcome Y through health status L. U1 represents unmeasured common causes of both L and Y. Exposure (E) is also connected with censoring, both directly and through unmeasured common causes (U2).
Potentially informative censoring (i.e., related to health status and exposure) was examined through the use of inverse probability censoring weighting. Inverse probability weights create a pseudopopulation in which, under a set of assumptions and correct model specification, there is no bias due to censoring. The target parameter in this pseudopopulation is the effect of exposure “had no one been censored,” which is unrealistic for occupational health studies if we treat normal retirement as censoring. Therefore, we chose to define censoring as termination of employment prior to the age of 55 years to capture premature retirement or voluntary quitting. We also excluded layoffs because they were assumed not to be caused by exposure. Unfortunately, we could not distinguish recession-related early retirements from those potentially caused by harmful exposure.
Exposure appeared to be protective for censoring, suggesting that lower-exposure jobs may have been more likely to be eliminated because of unmeasured economic or other industry-related factors. This finding should be interpreted with caution given the challenges in distinguishing informative from noninformative censoring, but informative censoring may be a source of bias in some occupational studies, and controlling for it may sometimes correct underestimates of exposure response. Censoring did not appear to be very influential in this study.
We observed a higher risk of IHD associated with occupational PM2.5 exposure among workers in the aluminum industry. We also observed a downward bias due to the HWSE in smelting facilities, leading to attenuation in traditional models compared with MSMs. Concentration and particle composition may account for differences in the magnitude of IHD risk associated with exposure in the 2 different work environments, but the evidence suggests a higher risk of IHD in relation to PM2.5 exposure in both smelters and fabricators.
ACKNOWLEDGMENTS
Author affiliations: Division of Environmental Health Sciences, University of California Berkeley School of Public Health, Berkeley, California (Andreas M. Neophytou, Sadie Costello, Daniel M. Brown, Sally Picciotto, Elizabeth M. Noth, S. Katharine Hammond, Ellen A. Eisen); and Department of Internal Medicine, Stanford University, Stanford, California (Mark R. Cullen).
This study was supported by the National Institutes of Health, Institute of Aging (grant R01-AG026291-01) and the Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health (grant R01 OH009939-01).
Note on National Institute of Aging data sharing: As an alternative to providing a deidentified data set to the public domain, we allow access for the purpose of reanalyses or appropriate “follow-on” analyses to any qualified investigator willing to sign a contractual covenant with the host institution limiting the use of data to a specific agreed-upon purpose and observing the same restrictions as are set forth in our contract with Alcoa, Inc., such as 60-day manuscript review for compliance purposes.
Conflict of interest: M.R.C. receives salary support from Alcoa, Inc. (Pittsburgh, Pennsylvania) through contracts with Stanford University (Stanford, California). S.K.H. has received compensation as a member of the scientific advisory board for Alcoa, Inc., in the past. She has also consulted for Alcoa, Inc., and received compensation.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 3.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 4.Dong GH, Qian ZM, Xaverius PK, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61(3):578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 5.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113(6):670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Pope CA, 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 8.Magari SR, Hauser R, Schwartz J, et al. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation. 2001;104(9):986–991. doi: 10.1161/hc3401.095038. [DOI] [PubMed] [Google Scholar]
- 9.Fang SC, Eisen EA, Cavallari JM, et al. Acute changes in vascular function among welders exposed to metal-rich particulate matter. Epidemiology. 2008;19(2):217–225. doi: 10.1097/EDE.0b013e31816334dc. [DOI] [PubMed] [Google Scholar]
- 10.Torén K, Bergdahl IA, Nilsson T, et al. Occupational exposure to particulate air pollution and mortality due to ischaemic heart disease and cerebrovascular disease. Occup Environ Med. 2007;64(8):515–519. doi: 10.1136/oem.2006.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang SC, Cassidy A, Christiani DC. A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int J Environ Res Public Health. 2010;7(4):1773–1806. doi: 10.3390/ijerph7041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen MR. Invited commentary: The search for preventable causes of cardiovascular disease—whither work? Am J Epidemiol. 2009;169(12):1422–1425. doi: 10.1093/aje/kwp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen EA, Robins JM, Picciotto S. Healthy worker effect. In: El-Shaarawi AH, Piegorsch W, editors. Encyclopedia of Environmetrics. 2nd ed. Chichester, United Kingdom: John Wiley & Sons, Ltd; 2012. pp. 1269–1272. [Google Scholar]
- 14.Costello S, Brown DM, Noth EM, et al. Incident ischemic heart disease and recent occupational exposure to particulate matter in an aluminum cohort. J Expo Sci Environ Epidemiol. 2014;24(1):82–88. doi: 10.1038/jes.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benke G, Abramson M, Sim M. Exposures in the alumina and primary aluminium industry: an historical review. Ann Occup Hyg. 1998;42(3):173–189. doi: 10.1016/s0003-4878(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 16.Rønneberg A. Mortality and cancer morbidity in workers from an aluminium smelter with prebaked carbon anodes—Part III: mortality from circulatory and respiratory diseases. Occup Environ Med. 1995;52(4):255–261. doi: 10.1136/oem.52.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavallari JM, Eisen EA, Fang SC, et al. PM2.5 metal exposures and nocturnal heart rate variability: a panel study of boilermaker construction workers. Environ Health. 2008;7:36. doi: 10.1186/1476-069X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friesen MC, Demers PA, Spinelli JJ, et al. Chronic and acute effects of coal tar pitch exposure and cardiopulmonary mortality among aluminum smelter workers. Am J Epidemiol. 2010;172(7):790–799. doi: 10.1093/aje/kwq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noth EM, Dixon-Ernst C, Liu S, et al. Development of a job-exposure matrix for exposure to total and fine particulate matter in the aluminum industry. J Expo Sci Environ Epidemiol. 2014;24(1):89–99. doi: 10.1038/jes.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevrier J, Picciotto S, Eisen EA. A comparison of standard methods with g-estimation of accelerated failure-time models to address the healthy-worker survivor effect: application in a cohort of autoworkers exposed to metalworking fluids. Epidemiology. 2012;23(2):212–219. doi: 10.1097/EDE.0b013e318245fc06. [DOI] [PubMed] [Google Scholar]
- 21.Park RM. Mortality at an automotive engine foundry and machining complex. J Occup Environ Med. 2001;43(5):483–493. doi: 10.1097/00043764-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Costello S, Garcia E, Hammond SK, et al. Ischemic heart disease mortality and PM3.5 in a cohort of autoworkers. Am J Ind Med. 2013;56(3):317–325. doi: 10.1002/ajim.22152. [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumas O, Le Moual N, Siroux V, et al. Work related asthma. A causal analysis controlling the healthy worker effect. Occup Environ Med. 2013;70(9):603–610. doi: 10.1136/oemed-2013-101362. [DOI] [PubMed] [Google Scholar]
- 28.Modrek S, Cullen MR. Health consequences of the ‘Great Recession’ on the employed: evidence from an industrial cohort in aluminum manufacturing. Soc Sci Med. 2013;92:105–113. doi: 10.1016/j.socscimed.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer FJ, Galusha D, Slade M, et al. Process of care compliance is associated with fewer diabetes complications. Am J Manag Care. 2014;20(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo J, Goldstein BA, Cantley LF, et al. Contribution of health status and prevalent chronic disease to individual risk for workplace injury in the manufacturing environment. Occup Environ Med. 2014;71(3):159–166. doi: 10.1136/oemed-2013-101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modrek S, Cullen MR. Job Demand and Early Retirement. Chestnut Hill, MA: Center for Retirement Research at Boston College; 2012. [Google Scholar]
- 32.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc. 1977;39(1):1–38. [Google Scholar]
- 33.Robins JM. Correction for non-compliance in equivalence trials. Stat Med. 1998;17(3):269–302. doi: 10.1002/(sici)1097-0258(19980215)17:3<269::aid-sim763>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Robins J. A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. J Chronic Dis. 1987;40(suppl 2):139S–161S. doi: 10.1016/s0021-9681(87)80018-8. [DOI] [PubMed] [Google Scholar]
- 35.Robins JM, Blevins D, Ritter G, et al. G-estimation of the effect of prophylaxis therapy for Pneumocystis carinii pneumonia on the survival of AIDS patients. Epidemiology. 1992;3(4):319–336. doi: 10.1097/00001648-199207000-00007. [erratum in Epidemiology. 1993;4(2):189] [DOI] [PubMed] [Google Scholar]
- 36.van der Laan MJ, Gruber S. Targeted minimum loss based estimation of causal effects of multiple time point interventions. Int J Biostat. 2012;8(1) doi: 10.1515/1557-4679.1370. [DOI] [PubMed] [Google Scholar]
- 37.Picciotto S, Brown DM, Chevrier J, et al. Healthy worker survivor bias: implications of truncating follow-up at employment termination. Occup Environ Med. 2013;70(10):736–742. doi: 10.1136/oemed-2012-101332. [DOI] [PubMed] [Google Scholar]
- 38.Picciotto S, Chevrier J, Balmes J, et al. Hypothetical interventions to limit metalworking fluid exposures and their effects on COPD mortality: g-estimation within a public health framework. Epidemiology. 2014;25(3):436–443. doi: 10.1097/EDE.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 39.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]


