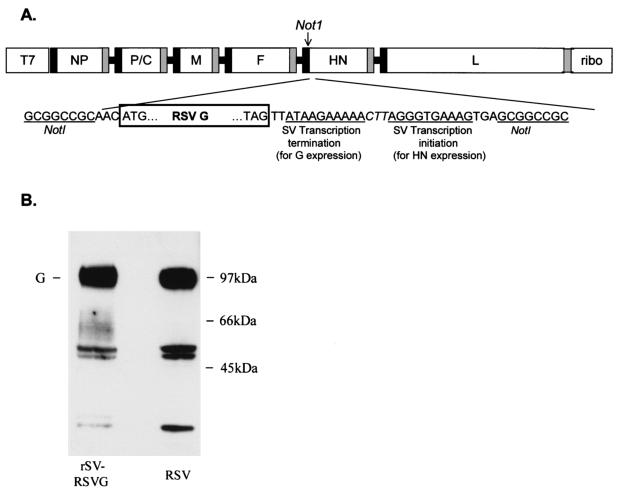
FIG. 1.
Design of pSV(+)RSVG and expression of RSV G target gene. (A) A unique NotI restriction enzyme site was created in the noncoding region of the HN gene to insert the RSV G glycoprotein gene. The NotI site was introduced into a subcloned ClaI-EcoRI fragment of pSeV(+) in pTF1 (21) by using a QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, Calif.). This modified fragment was then substituted for the wild-type fragment in pSeV(+) to create pSV(+)N. RSV G gene was cloned by using a forward primer which included a NotI site and a reverse primer which included an SV transcription termination signal and another transcription initiation signal (separated by an intergenic linker sequence [CTT]), followed by the NotI site. Thus, RSV G transcription initiated from the upstream SV HN transcription initiation sequence and terminated by using the new termination sequence. SV HN transcription initiated by using the newly introduced transcription initiation sequence. T7, T7 promoter; ribo, hepatitis delta virus ribozyme sequence. Black and gray boxes represent transcription initiation and termination sequences, respectively, of the nucleoprotein (NP), polymerase (P), matrix (M), fusion (F), hemagglutinin-neuraminidase (HN), and large (L) protein. (B) Western blot examination of lysates of HEp-2 cells (approximately 106 cells) infected with rSV RSVG (left lane) or wild-type RSV (right lane). Cells were lysed with 0.2 ml of TNE buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, and 1 mM EDTA) and were clarified (15,000 × g, 10 min). Supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in nonreducing conditions, transferred to Immobilon membrane (Millipore, Danvers, Mass.), and developed with RSV G-specific monoclonal antibody (clone 63-10F; Chemicon International Inc., Temecula, Calif.). Fully glycosylated RSV G protein (both N- and O-linked glycosylation) runs at approximately 90 kDa (G). Middle band likely represents partially glycosylated G protein, and the lower band represents unglycosylated G.