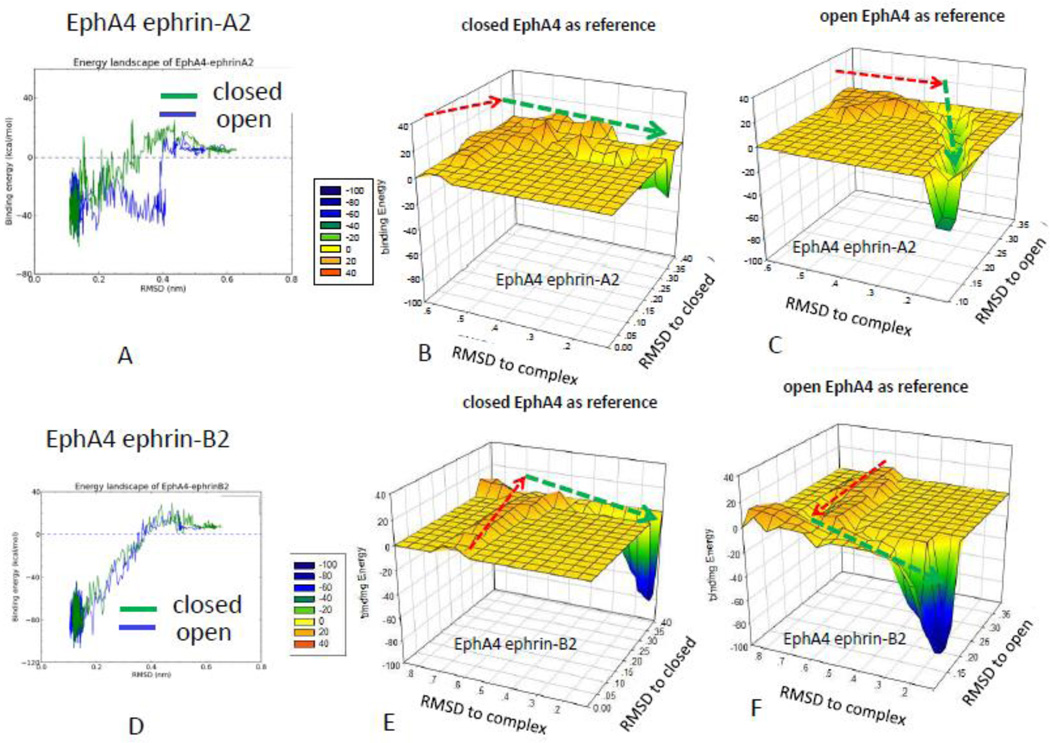
Figure 4.
Comparison of the energy landscape of the Eph-ephrin recognition starting with open and closed Eph conformations reveals that the EphA4 receptor uses a different mechanism to interact with ephrin-A2 and ephrin-B2. (A) Comparison of EphA4 ephrin-A2 interaction starting with closed and open EphA4 conformations. Starting from a closed EphA4 conformation, the RMSD with respect to the closed (B) and open (C) EphA4 conformations suggests simutanous ephrin-A2 recognition and adjustment to the open EphA4 conformation. (D) Comparison of EphA4 ephrin-B2 interaction starting with closed and open EphA4 conformations. Starting from a closed EphA4 conformation, the three-dimensional energy landscapes of the EphA4 ephrin-B2 interaction of the closed (E) and (F) open EphA4 conformations suggests that adjusting to the open EphA4 conformation takes place at the early stage of ephrin-B2 recognition.