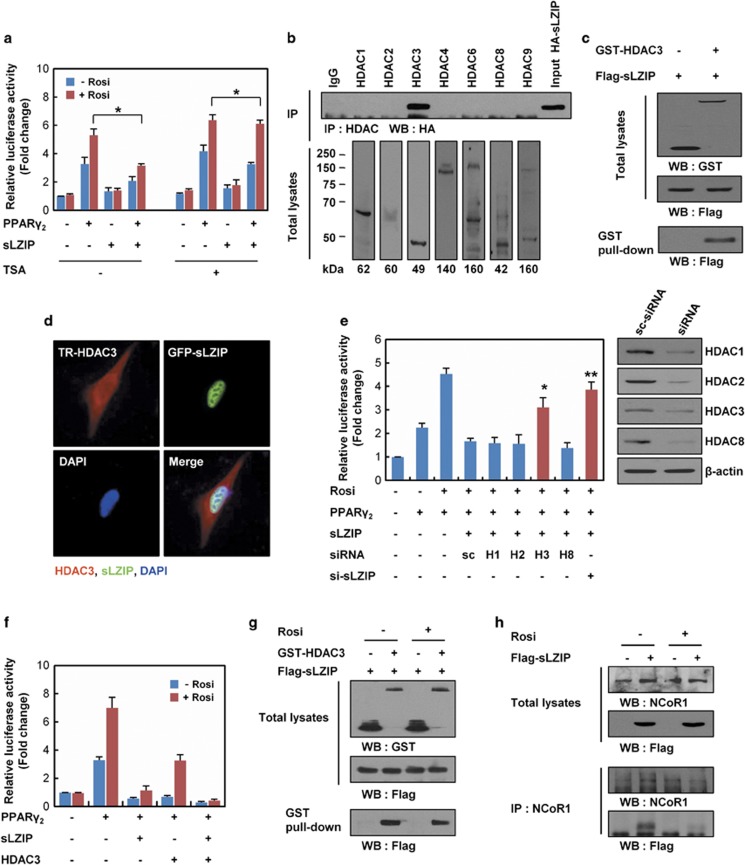
Figure 3.
HDAC3 is involved in sLZIP suppression of PPARγ2 transcriptional activity. (a) 293T cells were transfected with FABP4-Luc, β-galactosidase, PPARγ2, and sLZIP. Following transfection, cells were stimulated with or without 10 μM Rosi and 100 nM TSA. Promoter activity was determined by a luciferase assay. *P<0.005. (b) HA-sLZIP was transfected into 293T cells. Cell lysates were collected and immunoprecipitation assays were performed using the indicated antibodies. (c) GST-HDAC3 and Flag-sLZIP were co-transfected into 293T cells. Cell lysates were collected and GST pull-down assays were performed using glutathione 4B beads. (d) GFP-sLZIP were expressed into C3H10T1/2 cells and analyzed by fluorescence microscopy using Texas Red-conjugated anti-HDAC3 antibody. The nucleus was stained with DAPI. (e) 293T cells were transfected with FABP4-Luc, β-galactosidase, PPARγ2, sLZIP, and 100 nM siRNAs against scramble (sc), sLZIP, HDAC1, 2, 3, and 8 (H1, H2, H3, and H8, respectively). After transfection, cells were stimulated with or without 10 μM Rosi for 24 h, and promoter activity was determined. *P<0.01; **P<0.005. (f) 293T cells were co-transfected with FABP4-Luc, β-galactosidase, PPARγ2, HDAC3, and sLZIP. Following transfection, cells were stimulated with or without 10 μM Rosi for 24 h and subjected to a luciferase assay. Luciferase activities were normalized to the β-galactosidase activity and presented as the relative activity. (g) 293T cells were transfected with GST-HDAC3 and Flag-sLZIP, and treated with or without 10 μM Rosi for 24 h. Cell lysates were subjected to a GST pull-down assay. (h) Flag-sLZIP was expressed into 293T cells. Cells were treated with 10 μM Rosi for 24 h. Cell extracts were analyzed by an immunoprecipitation assay using an anti-NCoR1 antibody. Protein complex was analyzed on SDS-PAGE, followed by western blotting