Figure 4. The FXI A3 Domain and fIX Gla-domain.
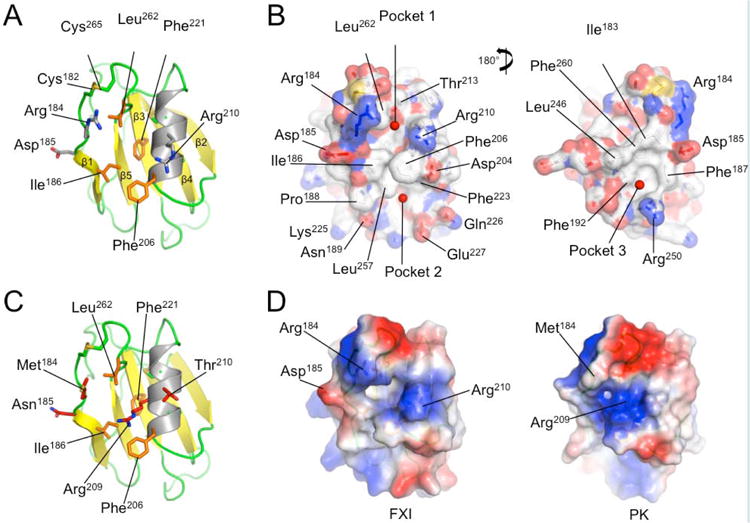
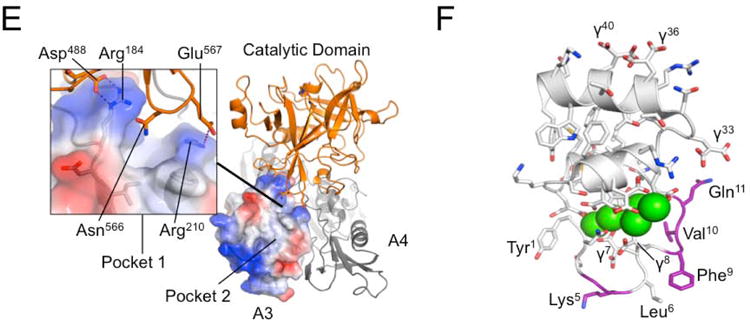
(A) Topological diagram showing the A3 domain of human fXI with the β-sheet indicated in yellow and α-helix in grey. Side chains of residues in the locality of Arg184 are shown as sticks. (B) Surface representations of the fXI A3 domain are shown with partial transparency to highlight side chains of specific residues that are colored by atom type. Two rotations are shown related by 180 degrees. The software program Metapocket identified three pockets on the surface of A3 (shown as red balls) (C) Topological diagram of the human PK A3 domain model with side chains of non-conserved residues in the area of the pocket in fXI shown as sticks. Note that the side chain of Arg209 in PK occupies the site of the hydrophobic pocket in fXI. (D) Charged surface representations of fXI (left) and PK (right) A3 domains. Blue indicates positive charge and red negative charge. Note the absence of pocket 1 in the PK A3 domain model due to the Arg209 side chain. (E) Structure of the zymogen fXI monomer showing the A3 domain as a charged surface representation, and the A4 and catalytic domains as ribbon drawings. Note that Arg184 and the adjacent hydrophobic pocket 1 are covered by the catalytic domain. The inset shows specific interactions between side chains of the protease domain and A3. (F) Topological diagram of the human fIX Gla-domain with residues in the Ω-loop (4 to 11) that differ from the corresponding region of the human fVII Gla-domain highlighted in magenta. Positions of certain γ-carboxyglutamic acid residues are indicated by the symbol “γ”. Calcium ions in the vicinity of the Ω-loop are represented by green spheres. The image is derived from a structure for a complex between the human fIX Gla-domain and the antibody 10C12.29 Figures prepared with Pymol (The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC. http://www.pymol.org/citing).
