Summary
Background
We hypothesize that perinatal exposures, in particular the human microbiome and maternal nutrition during pregnancy, interact with the genetic predisposition to cause an abnormal immune modulation in early life towards a trajectory to chronic inflammatory diseases such as asthma and others.
Objective
The aim of this study is to explore these interactions by conducting a longitudinal study in an unselected cohort of pregnant women and their offspring with emphasis on deep clinical phenotyping, exposure assessment, and biobanking. Exposure assessments focus on the human microbiome. Nutritional intervention during pregnancy in randomized controlled trials are included in the study to prevent disease and to be able to establish causal relationships.
Methods
Pregnant women from eastern Denmark were invited during 2008–2010 to a novel unselected ‘COPSAC2010’ cohort. The women visited the clinic during pregnancy weeks 24 and 36. Their children were followed at the clinic with deep phenotyping and collection of biological samples at nine regular visits until the age of 3 and at acute symptoms. Randomized controlled trials of high‐dose vitamin D and fish oil supplements were conducted during pregnancy, and a trial of azithromycin for acute lung symptoms was conducted in the children with recurrent wheeze.
Results
Seven hundred and thirty‐eight mothers were recruited from week 24 of gestation, and 700 of their children were included in the birth cohort. The cohort has an over‐representation of atopic parents. The participant satisfaction was high and the adherence equally high with 685 children (98%) attending the 1 year clinic visit and 667 children (95%) attending the 2 year clinic visit.
Conclusions
The COPSAC2010 birth cohort study provides longitudinal clinical follow‐up with highly specific end‐points, exposure assessments, and biobanking. The cohort has a high adherence rate promising strong data to elucidate the interaction between genomics and the exposome in perinatal life leading to lifestyle‐related chronic inflammatory disorders such as asthma.
Keywords: Asthma, eczema, allergy, child, birth cohort, microbiome, vitamin D, fish oil, randomized clinical trial
Background
Asthma, eczema, and allergy are the most common chronic diseases in childhood and the major cause for healthcare utilization 1. These diseases are rarely life‐threatening, but severely affect the quality of life of children and their families, in particular families with young children.
The prevalence of childhood asthma, eczema, and allergy has increased several fold in recent decades in westernized cultures, suggesting a strong influence of environmental factors on disease expression 2. Diseases with such dramatic increase despite a generally improved welfare and health care represent a significant societal and scientific challenge. With a stable genetic pool, the changing geographical and temporal incidences offer evidence of important, yet unknown, lifestyle changes causing a new category of welfare diseases. Particularly, environmental exposures in pre‐natal and perinatal life, including the early microbiome 4 and maternal vitamin D 6 and fish oil 7 intake during pregnancy, have been hypothesized to affect the risk of asthma, eczema, and allergy. Risk factors and pathways seem to be shared with other chronic inflammatory diseases later in life [8] meaning that understanding of the mechanisms causing asthma, eczema and allergy may have implications for a wider range of lifestyle related inflammatory disorders.
The mission of the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) is to develop new knowledge on the inception of asthma, allergy, and eczema in order to provide a basis for primary and secondary preventive measures, novel diagnostic tests, and therapeutics for these chronic diseases. COPSAC is fundamentally a prospective clinical study of pregnancy cohorts following the children with an intense focus on longitudinal deep phenotyping in disease and health together with genotyping and objective assessments of the exposome.
The first COPSAC birth cohort was built around year 2000 (COPSAC2000) of 411 children of mothers with a history of asthma 9. This at‐risk cohort has generated substantial new insights into the origins of asthma, eczema, and allergy. Ten years later, COPSAC has built a second birth cohort (COPSAC2010) on the routines and experiences of the first COPSAC2000 birth cohort. The aim is to replicate and explore key findings from COPSAC2000 in an unselected population. Randomized controlled trials of high‐dose vitamin D and fish oil supplements were conducted during pregnancy, and a trial of azithromycin for acute lung symptoms was conducted in the children with early asthma symptoms. These interventions were included to prevent and treat disease and to be able to establish causal relationships behind previously observed associations. The main hypotheses are as follows:
Chronic inflammatory disorders like asthma are programmed in the perinatal life as the results of complex gene–environment interactions.
The early human microbiome is an important environmental exposure, where an unbalanced composition may cause deregulation of the immature immune system leading to a trajectory towards chronic inflammatory diseases such as asthma.
Maternal diet during pregnancy influence maturation and regulation of neonatal immune responses and provide an opportunity for preventive supplementation with vitamin D and n‐3 long‐chain polyunsaturated fatty acids (n‐3 LCPUFA) in pregnancy.
Asthma, eczema, and allergies represent several specific endotypes, each associated with divergent underlying molecular mechanisms, distinct clinical features, and individual treatment responses. Identifying these endotypes is essential for establishing effective preventive measures and determining appropriate individualized treatment.
This article describes the recruitment, baseline, clinical study programme, and the standard operating procedures built for the COPSAC2010 birth cohort. The aim is to share methods and hypotheses of the COPSAC resource that will generate important scientific discoveries in the future and to invite international collaborators to explore this vast biobank and metadata.
Materials and methods
COPSAC2010 pregnancy cohort
Recruitment
The study catchment area was Zealand, an island in the eastern part of Denmark, including the capital Copenhagen. Pregnant women were recruited by a monthly surveillance of reimbursement to general practitioners for the mandatory pregnancy visit. They received an invitation by posted mail to contact the clinic during 2008–2010. Exclusion criteria were gestational age above week 26; daily intake of more than 600 IU vitamin D during pregnancy; or having any endocrine, heart, or kidney disorders. Women who contacted the COPSAC clinic by phone received detailed verbal information. Those who were still interested and qualifying for the study received comprehensive study information by posted mail. Finally, the women attended the clinical research unit within pregnancy weeks 22–26 for a visit in the research clinicwith detailed information and enrolment into the pregnancy cohort.
Power calculation
The cohort was powered for replication of the association between neonatal airway colonization and early asthma symptoms as discovered in COPSAC2000 4. The main outcome was ‘recurrent wheeze’, based upon an algorithm of recurrent troublesome lung symptoms as previously described [10]. Power calculations were based on survival analysis (log‐rank test). Based on the findings from COPSAC2000, we assumed that 12% of children would develop ‘recurrent wheeze’ in the first 3 years of life (16.5% in the COPSAC2000 high‐risk cohort). Furthermore, the following were assumed: the prevalence of colonization in infancy to be 20% (21% in COPSAC2000); a hazard ratio of 2 associated with colonization (2.40 in COPSAC2000); an exponential survival curve; and a dropout rate of 5% of recruited pregnant women and of further 15% of children by age 3 years. With a significance level of 0.05 and a power of 80%, the required sample size would be 660 recruited pregnant women. Statistical power for the randomized trials was calculated based on the available numbers. Six hundred and ninety‐eight newborns were included in the fish oil study and 587 in the vitamin D study. With an assumed hazard ratio of 0.5 in the treatment arms and expected frequency of ‘recurrent wheeze’ of 12% in the control arm, this resulted in a power of 73% for detection of a statistically significant difference with a significance level of 0.05. Statistical power in the vitamin D study following similar calculations was 65%.
Selection analysis
Characteristics of the women not included in the pregnancy cohort were analysed from letters sent to 1000 women, who had not responded to the primary invitation, inviting them to answer a number of general health questions. Reminder letters were sent on two occasions to non‐responders.
COPSAC2010 birth cohort
Neonates born to the women from the COPSAC2010 pregnancy cohort were included in the COPSAC2010 birth cohort.
Scheduled visits were planned at 1 week, 1, 3, 6, 12, 18, 24, 30, and 36 months, and regularly thereafter until age 7.
Acute visits were arranged whenever parents considered the need for medical attention for significant troublesome lung symptoms or at the onset or exacerbation of eczema. Asthma, eczema, allergic rhinitis and respiratory infections were diagnosed and treated by the doctors in the research clinic according to predefined algorithms (detailed in the Supporting information).
Database
All information was obtained by personal interview at clinical visits by medical doctors (MDs) and research assistants both with paediatric training. Medical, familial, environmental, and socio‐economic histories were assessed by predefined questions and closed response categories and entered online into a dedicated database running an audit trail (Oracle database on a Novel SQL server). Objective information and measurements were entered into the database, subsequently double‐checked against source data, and finally locked for further editing. Quality assurance followed ‘Good Clinical Practice’ guidelines (detailed in the Supporting information).
Standard operating procedures (SOPs) were predefined for all study and database registration procedures similar to those used in the ongoing at‐risk COPSAC2000 birth cohort study (see Supporting information) and were available online and in binders in every clinic room.
Participant communication
Telephone calls, posted mails, emails, text messages, and Internet survey (www.esurveyspro.com) were used to keep a close and continuing communication with the participating families between the visits.
Nested, blinded, controlled, randomized clinical trials
Vitamin D supplement during third pregnancy trimester
The pregnant women were randomized 1 : 1 to a daily dose of 2400 IU cholecalciferol D3 daily supplement or matching placebo (Camette, Denmark A/S) from pregnancy week 24 to 1 week after delivery. All participants were instructed to continue supplementation of 400 IU cholecalciferol D3 currently recommended by the Danish National Board of Health; that is, daily doses were 400 IU vs. 2800 IU. Serum vitamin D level was assessed at the time of randomization and at completion allowing for stratification of habitual vitamin D intake and assessment of adherence to the treatment plan. Counting returned capsules complemented assessment of compliance. The primary end‐point is the development of ‘recurrent wheeze’, based upon an algorithm of episodes of troublesome lung symptoms, and will be analysed when all the participating children reach 3 years of age.
Fish oil supplement during third pregnancy trimester
From pregancy week 24 to 1 week after delivery, the pregnant women were randomized 1 : 1 to daily fish oil supplement (four 1‐g capsules (Incromega TG33/22) supplying a total of 2400 mg/day of n‐3 LCPUFA; 1320 mg/day eicosapentaenoic acid (EPA), and 880 mg/day docosahexaenoic acid (DHA) contributing to an estimated 10‐fold increase relative to the normal daily intake of n‐3 LCPUFA or a matching placebo (four 1‐g capsules of olive oil (Pharmatech A/S, Norway) containing 72% n‐9 oleic acid and 12% n‐6 linoleic acid, which contributes an estimated 3% of normal daily intake of PUFA. Whole‐blood PUFA levels were assessed at the time of randomization and at completion allowing for stratification of habitual n‐3 LCPUFA intake and assessment of adherence to the treatment plan. Compliance was assessed by counting returned capsules. The primary end‐point is the development of ‘recurrent wheeze’, based upon an algorithm of episodes of troublesome lung symptoms and will be analysed when all participating children reach 3 years of age.
Azithromycin treatment of acute severe episodes of troublesome lung symptoms
The children aged 12–36 months with recurrent episodes of troublesome lung symptoms lasting at least 3 days were offered participation in a double‐blinded, randomized, placebo‐controlled treatment with azithromycin or placebo at acute episodes of troublesome lung symptoms. Children were recruited from the main cohort study if they had recurrent ’wheezy episodes’ (five episodes of three consecutive days of troublesome lung symptoms within a period of 6 months or 4 weeks of continuous troublesome lung symptoms or acute severe asthma). At every subsequent episode, treatment was administered at acute clinical visits, after excluding suspected pneumonia (tachypnoea, RF ≥50; fever ≥39°C; CRP ≥50). The study was designed as a double‐blind, placebo‐controlled, randomized, cross‐over comparison of azithromycin in a dose of 10 mg/kg/day (TEVA, Kgs. Lyngby, Denmark A/S) for 3 days or matching placebo (the Pharmacy of Glostrup, Copenhagen). Randomization of the trials was performed at the Pharmacy of Glostrup, Copenhagen, and copies of the randomization code were kept in sealed envelope at the research site as well as at the pharmacy. The primary end‐points are symptom burden and duration of the asthmatic episode after randomization and will be analysed when a total of 250 treatments have been completed or all children turned 3 years of age.
Figure S9 schematically shows the relationship between the trials on nutritional intervention during pregnancy and the trial on azithromycin.
Influenza A H1N1v vaccination
During the H1N1v pandemic winter 2009–2010, the women were invited from gestational week 20 until 8 months after birth to a phase IV randomized, participant‐blinded comparison of monovalent influenza A/California/2009 (H1N1v) surface antigen vaccine (Focetria®, Novartis Vaccines and Diagnostics GmbH, Marburg, Germany) in both MF59‐adjuvanted and non‐adjuvanted forms 11. The study consisted of four groups: pregnant women receiving: (1) 7.5 µg H1N1pnd09 antigen with a full complement of MF59 adjuvant (P7.5 µg); (2) 3.75 µg antigen, with half the usual content of MF59 (P3.75 µg); (3) 15 µg antigen, unadjuvanted vaccine (P15 µg); and (4) non‐pregnant women receiving 7.5 µg antigen with full MF59‐adjuvanted vaccine (NP7.5 µg). After recruitment, the pregnant women were randomized by a computer‐generated permuted block randomization table, in blocks of 45 women with allocation rate of 1 : 1 : 1. The P3.75 µg group was closed after 4 months due to low recruitment rate.
Clinical assessments
Daily diary cards were used from birth to monitor significant troublesome lung symptoms as previously analysed in detail 12 including components of cough, wheeze, and dyspnoea, and use of β2‐agonists, inhaled corticosteroids, and montelukast. Skin symptoms were monitored as active eczema and use of topical steroids. In addition, the diary cards monitored infections, categorized into common cold, pneumonia, pharyngitis, otitis, fever, gastrointestinal infection, and absence from day care institution because of illness (Figure S1). The diary cards were reviewed with the family by the research MD at each visit to validate symptom definitions. All information were subsequently entered into the online database and double‐checked.
Physical examination was performed by the research MD at all scheduled and acute visits, including lung and heart stethoscopy and examination of skin 13, ear, nose, and throat.
CRP measurement was performed at acute visits or when needed using QuickRead 101 (QuickRead Instrument, Orion Diagnostica, Espoo, Finland).
Tympanometric evaluation of the middle ear pressure was performed at yearly visits or when needed (MT10, Interacoustics, Denmark) on both ears.
Spontaneous physical activity was assessed by age 2 years using an omnidirectional accelerometer worn on the ankle for 2 weeks as previously described 16.
Blood pressure was assessed yearly from age 3 years (Welch Allyn Connex: ProBP 3400).
Lung function by multiple‐breath washout (EcoMedics: Exhalyzer D) was assessed from the age of 3 years.
Airway resistance was measured from 3 years of age by whole‐body plethysmography (Master Screen Body; Erich Jaeger GmbH; Würzburg, Germany) 18.
Airflow was measured by spirometry (Vitalograph: Spirotrac II) before and after inhalation of a standard dose of β2‐agonist from age 5 years.
Fractional nitric oxide (FeNO) was measured using an Aerocrine NO system (CLG77AM chemiluminescence analyzer from Ecophysics AG, Duernten, Switzerland) and assessed from age 5 years.
Anthropometrics
Gestational age and fetal biometrics were determined by an ultrasound nuchal translucency scan in week 13 of the pregnancy (biparietal diameter and occipito‐frontal diameter, abdominal circumference, length of femur, and fetal weight estimated by the Hadlock equation for fetuses <2500 g) 20.
Length was measured at every visit until age 24 months by the infantometer, Kiddimetre® (Raven Equipment Limited, Essex England).
Height was measured at every visit from age 24 months (i.e. overlapping with length at age 24 months) by Harpenden stadiometer (Holtain Ltd, Crymych, Dyfed, Wales).
Parental height was measured at recruitment to the study with Harpenden stadiometer.
Weight was measured at every visit using calibrated digital weight scales.
Head circumference was measured three times at each visit to the closest millimetre at the frontal and occipital protuberance positions.
Abdominal circumference was measured three times at each visit at the level of the umbilicus, in the horizontal parallax.
Whole‐body dual‐energy X‐ray absorptiometry (DXA, GE Healthcare, Fairfield, CT, USA) was measured at age 3 years for detailed body composition.
Motor and cognitive development
Milestones of motor development were monitored prospectively by the parents using a registration form based on The Denver Development Index and WHO milestones registration 21.
Language development was assessed at 12 and 24 months of age by a web‐based parent report instrument (MacArthur CDI form) translated into Danish 23.
Cognitive development was assessed at 30 months using the cognitive part of Bayley Scales of Infant and Toddler Development, third edition 24. The tests were performed by trained examiners, and all sessions were video recorded allowing reviewing by a single observer.
Immune function
Mucosal lining fluids were sampled from the upper airways at 1 and 24 months of age as well as at all acute airway visits for assessment of cytokines and chemokines as previously described 25.
At 18‐months, whole blood samples were stimulated with Toll‐like receptor (TLR) ligands, cell type‐specific ligands, and a NLRP3 inflammasome ligand. Whole‐blood pheno‐typing by flow cytometry was performed at 18 months and in a random subset of cord blood samples.
Skin prick tests with milk, egg, dog, and cat extracts were performed at 6 and 18 months, using standard extracts from ALK‐ABELLO with both a positive control and a negative control.
Specific IgE was determined at 6 and 18 months by ImmunoCAP (Pharmacia Diagnostics AB, Uppsala, Sweden) against 15 common inhalant and food allergens (cat, dog, horse, birch, timothy grass, mugwort, house dust mites, moulds, hen's egg, cow's milk, fish, wheat, peanut, soybean, and shrimp).
Genomics
Deoxyribonucleic acid was purified from peripheral blood cell fraction from plasma of the infant collected at 6 months and from both parents at recruitment and stored at −80°C.
Genome‐wide genotyping was performed using the Illumina OmniExpress and Exome beadchip and imputed using both the Hapmap and 1000 genomes reference populations 28.
RNA was stabilized in the blood sample collected at 18 months and stored at −80°C.
Biobanking
Cord blood was collected by needle puncture from the umbilical cord immediately after birth (collected by midwives) and transported to the laboratory by courier, where cells, plasma, and serum were separated and stored.
Neonatal dried blood spots were sampled as part of the neonatal screening programme and stored in the Danish Neonatal Screening Biobank for further analyses of metabonomics, epigenetic alterations 30 and cytokine levels 31.
Exhaled volatile organic compounds (VOCs) were assessed at 1 and 18 months of age and at acute visits. Breath samples collected were analysed by an ‘Electronic nose’ (Cyranose 320) with a 32‐carbon sensor array for assessment of fingerprints, and specific VOCs were measured by gas chromatography–mass spectrometry.
Urine was sampled at 1 and 12 months of age and stored at −80°C for biomarker analyses.
Peripheral blood was collected by 6 and 18 months of age and stored as plasma and serum and as peripheral blood mononuclear cells at 18 months.
Environmental exposures
History of exposures was recorded yearly from clinical interviews according to predefined categories including variables on social status (educational and occupational status of the parents and household income), siblings, and home environment (e.g. exposure to pets, passive smoking, and fireplace at home).
Adherence to asthma medication and use of antibiotics and other medications was verified from the online national register on medications filed at Danish pharmacies.
Hair samples were obtained at 12, 24, and 36 months for nicotine and cotinine assessment 32.
Dust samples from bedding were collected at 6 months of age by the parents. Settling dust was collected from the child's bedroom.
Human microbiome
Vaginal swaps were collected at 24 weeks and 36 weeks of pregnancy. Wet smears and conventional culture for bacteria were performed, and material was stored for full sequencing of the microbiome.
Hypopharyngeal aspirates were collected at 1 week, 1 month, and 3 months after birth, and at every acute visit with lung symptoms 33. The samples were cultured for conventional bacteria and stored for sequencing of the microbiome.
Nasopharyngeal aspirates were collected at 1 week, 1 month, 3 months, and at every acute visit with lung symptoms. Samples were stored for analysis of respiratory viruses.
Fecal samples were collected at 1 week, 1 month, and 12 months. The samples were cultured for conventional bacteria and stored for sequencing of the microbiome.
Skin swaps were performed at 12 months of age and again at 36 months from the volar forearm and the axilla and stored for sequencing of the microbiome.
Diet
Mother's diet was assessed from a 360‐item questionnaire with detailed information on dietary habits for the first month prior to recruitment 35.
Breast milk samples were obtained from the mother 1 month post‐partum. 0.01% 2,6‐di‐tert‐butyl‐4‐methylphenol was added to the milk and stored at −80°C for later fatty acid analysis 36.
Maternal blood fatty acid levels
EDTA blood samples were collected from the women in pregnancy week 24 and 1 week post‐partum, 0.01% 2,6‐di‐tert‐butyl‐4‐methylphenol was added, and the sample was stored at −80°C. Fatty acid analyses were performed within a year from sampling by gas chromatography 37.
Approvals
The main study protocol was approved by the Ethics Committee (H‐B‐2008‐093) and the Danish Data Protection Agency (2008‐41‐2599).
Vitamin D supplement randomized controlled trial in pregnant women: Ethics Committee (H‐B‐2009‐014); Danish Data Protection Agency (2008‐41‐2599); EudraCT number 2008‐007871‐26; ClinTrial.gov ID NCT00798226.
n‐3 LCPUFA supplement randomized controlled trial in pregnant women: Ethics Committee (H‐B‐2008‐093); Danish Data Protection Agency (2008‐41‐2599); ClinTrial.gov ID NCT00798226.
Azithromycin randomized controlled trial: Ethics Committee (H‐3‐2010‐065); Danish Data Protection Agency (2010‐41‐5023); EudraCT number 2010‐018592‐16. ClinTrial.gov ID NCT01233297.
Influenza A H1N1v vaccination randomized controlled trail: Ethics Committee (H‐B‐2008‐093); Danish Data Protection Agency (2009‐41‐4031); EudraCT number 2009‐016877‐14; ClinTrial.gov ID NCT01012557.
Results
Pregnancy cohort recruitment
Brief invitations were posted to 54 358 women. 1876 women responded and received further information. 733 women attended the clinical research unit for extensive information and were all included in the pregnancy cohort as illustrated in the flow chart of the study recruitment (Fig. 1). The 733 women participated during a total of 738 pregnancies as five women participated during two pregnancies. Questionnaires were mailed to 1000 women, who had not responded to the study invitation, of whom 870 (87%) women responded to the questionnaire.
Figure 1.
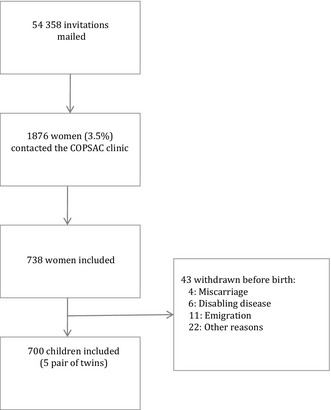
Flow of subjects from invitation to the first visit to the COPSAC clinic.
Table 1 compares the populations of 690 mothers participating in the birth cohort with the group of 870 women choosing not to participate. Women who chose to participate in the cohort were characterized by higher prevalence of asthma, eczema, and hayfever. They were more frequently employed as professional and less frequently unemployed, and had higher income levels. There were no differences with respect to tobacco smoking, alcohol intake, pets at home, or mode of delivery.
Table 1. Dropout analysis. Results from questionnaires mailed to 1000 women who had received an invitation during pregnancy, but chosen not to participate in the COPSAC2010 pregnancy cohort, compared with mothers of children participating in the study. The questionnaire response rate was 86%.
| Non‐participants, N = 870a | COPSAC2010 N = 690b | P‐value | |
|---|---|---|---|
| Natural birth | 82% | 78% | 0.098 |
| Furred pets in the home | 36% | 38% | 0.458 |
| Smoking during pregnancy, more than one cigarette per week | 9% | 7% | 0.256 |
| Alcohol during pregnancy, more than one unit per week | 2% | 2% | 0.697 |
| Asthma (self‐reported) | 11% | 30% | <0.001 |
| Eczema (self‐reported) | 12% | 21% | <0.001 |
| Allergy (self‐reported) | 27% | 34% | <0.0001 |
| Hayfever (self‐reported) | 20% | 38% | <0.0001 |
| Education level, university education longer than 3 years | 33% | 36% | 0.18 |
| Employment | 0.006 | ||
| Unemployed | 10% | 6% | – |
| Student | 8% | 8% | – |
| Non‐professional | 8% | 9% | – |
| Professional | 73% | 78% | – |
| Household income | <0.001 | ||
| Below 400 000 DKK | 22% | 12% | – |
| 400 000 DKK–600 000 DKK | 27% | 22% | – |
| 600 000–800 000 DKK | 27% | 29% | – |
| Above 800 000 DKK | 24% | 37% | – |
The response rate for the different variables varied from 849 to 870 of 870 non‐participants.
The response rate for the different variables varied from 620 to 690 of 690 participants.
Significant associations (P < 0.05) were marked in bold.
Birth cohort
Forty‐three children were withdrawn before enrolment into the birth cohort (Fig. 1; six disabling diseases, four deaths, 11 emigrations, 22 unspecified reasons). The birth cohort enrolled a total of 700 newborns at 1 week of age (born between 3 November 2008 and 17 March 2011), including five pairs of twins and five pairs of siblings born of a total of 690 mothers (Fig. 1). Baseline characteristics of the 700 children in the COPSAC2010 birth cohort are presented in Table 2 and compared with the previous COPSAC2000 birth cohort. The two cohorts are quite similar (the predefined difference in asthma risk aside), except for a remarkable reduction in mothers’ smoking and intake of alcohol during pregnancy, and a higher level of education in the recent COPSAC2010 cohort.
Table 2. Baseline characteristics of the COPSAC2010 cohort and comparison with COPSAC2000.
| Probands | COPSAC2010 | COPSAC2000 |
|---|---|---|
| Mothers enrolled, n | 738a | 452 |
| Number of newborns, n | 700b | 411c |
| Birth cohort | ||
| Boys | 51.4% | 49.4% |
| Twin pairs | 1% | 2% |
| Sibling pairs | 1% | 2% |
| Caucasian | 96% | 97% |
| Mother's age at birth, mean (SD), years | 32.2 (4.4) | 30.0 (4.5) |
| Father's age at birth, mean (SD), years | 34.4 (5.6) | 32.0 (5.2) |
| Season of birth | ||
| Winter | 31% | 23% |
| Spring | 27% | 21% |
| Summer | 21% | 27% |
| Fall | 21% | 29% |
| Pregnancy and birth | ||
| Assisted reproductive technology | 10% | |
| Gestational age, mean (SD), weeks | 39.9 (1.7) | 39.9 (1.6) |
| Birthweight, mean (SD), kg | 3.54 (0.555) | 3.52 (0.519) |
| Birth length, mean (SD), cm | 51.9 (2.5) | 52.3 (2.3) |
| Head circumference at 1 week, mean (SD), cm | 35.7 (1.4) | 35.2 (1.6) |
| Apgar at 5 min., mean (SD) | 9.9 (0.34) | 9.8 (0.6) |
| Mode of delivery, caesarean section, | 22% | 21% |
| Acute | 12% | 12% |
| Elective | 9% | 9% |
| Newborns hospitalized in relation to labour | ||
| Preterm birth (<36 weeks) | 3% | Excluded |
| In need of assisted ventilation | 2% | Excluded |
| Other reasons | 4% | 2% |
| Mothers hospitalized in relation to labour | 12% | |
| Exposures | ||
| Parity | ||
| 1 | 46% | 46% |
| 2 | 38% | 26% |
| 3 or more | 16% | 28% |
| Older children in household | ||
| 0 | 43% | 64% |
| 1 | 38% | 24% |
| 2 | 15% | 8% |
| 3 or more | 4% | 3% |
| Mother smoking during pregnancy | 8% | 24% |
| Alcohol use during pregnancy (any) | 14% | 26% |
| Antibiotics during pregnancy (any) | 37% | |
| Antibiotics during birth (any) | 33% | |
| Furred pets at home (any) | 37% | 30% |
| Duration of solely breastfeeding, mean (SD), days | 105 (62) | 113 (62) |
| Duration of breastfeeding, mean (SD), days | 240 (144) | 246 (156) |
| Socio‐economic variables | ||
|
Household annual income Index 2010. National average household income for families in 2010: 458 527 DKr | ||
| Below 400 000 DKK | 10% | 21% |
| 400 000–600 000 DKK | 24% | 31% |
| 600 000–800 000 DKK | 29% | 30% |
| Above 800 000 DKK | 37% | 17% |
| Mothers with university education (more than 3 years) | 28% | 13% |
| Fathers with university education (more than 3 years) | 28% | 17% |
| Mothers without occupation (unemployed or student) | 13% | 19% |
| Fathers without occupation (unemployed or student) | 8% | 7% |
| Atopic disposition (diagnosed by doctor) | ||
| Mothers with asthma | 26% | 100% |
| Mothers with allergic rhinitis | 30% | 73% |
| Mothers with eczema | 27% | 46% |
| Fathers with asthma | 21% | 15% |
| Fathers with allergic rhinitis | 27% | 30% |
| Fathers with eczema | 15% | 11% |
Mothers were recruited during 738 unique pregnancies, but these only represent 733 women as five women participated with more than one child (five sibling pairs participating in the cohort).
The response rate for the different variables varied from 680 to 700 of 700 children.
The response rate for the different variables varied from 385 to 411 of 411 children.
Adherence
The adherence to the study was high with 685 children (98%) attending the 1 year visit and 667 children (95%) attending the 2 year visit.
Cohort satisfaction with study participation
Table 3 shows results from the Internet survey of participant's satisfaction with the study. The main complaint was difficult parking access (27%) and, to a lesser extent, blood sampling in the infants (9%).
Table 3. Internet‐based survey of COPSAC2010 participants conducted in the period from 22 June 2011 to 5 January 2012. 618 responders of 690 study families.
| Study convenience | Unsatisfied | Satisfied | Very satisfied | N answered |
|---|---|---|---|---|
| Access to unscheduled visits | 3% | 49% | 48% | 612 |
| Regular examinations by doctor | 1% | 38% | 61% | 618 |
| Information on planned investigations | 1% | 32% | 67% | 618 |
| Burden of study visits and procedures | 1% | 54% | 45% | 614 |
| Continuity of research personnel | 6% | 51% | 43% | 618 |
| Atmosphere at the research clinic | 0% | 18% | 82% | 615 |
| Waiting time in the research clinic | 0% | 34% | 66% | 613 |
| Parking accessibility | 27% | 60% | 13% | 618 |
| Study procedures | Unsatisfied | Satisfied | Very satisfied | N participated |
|---|---|---|---|---|
| Blood sampling in parents | 1% | 37% | 62% | 609 |
| Blood sampling in child | 9% | 43% | 48% | 565 |
| Mucosal lining fluid sampling in child | 2% | 45% | 53% | 605 |
| Hypopharyngeal aspiration in child | 2% | 47% | 51% | 608 |
| VOC sampling in exhaled air | 2% | 43% | 55% | 601 |
| Accelerometer fixed for 2 weeks on the ankle of the child | 2% | 53% | 45% | 207 |
Discussion
The aim of COPSAC is to understand the origins of chronic inflammatory diseases with a primary focus on asthma, eczema and allergy in young children and to translate this into improved disease prevention, diagnosis, and treatment; to identify endotypes of diseases in early life from their disease mechanism; and specifically to understand the impact of the early human microbiome and maternal diet on the early immune maturation, disease development, and the interaction between the exposures and the genome.
Clinical birth cohorts
Information based on diagnoses and treatments of young children decided by community doctors and hospital records is of very low accuracy and precision. This is partly due to the fact that clinical assessments, diagnoses, and treatments are more difficult and imprecise in young children than later in life. This is of particular importance in young children with asthma, eczema, and allergy where there is very little standardization of diagnoses and treatments in the general medical community both nationally and internationally 1. Therefore, the COPSAC cohort studies of pregnant women and their children were planned as longitudinal clinical monitoring of symptoms, objective end‐points, and environmental exposures at a clinical research site with a standardized approach to diagnosis and treatment 9. The deep phenotyping includes prospective daily symptom characteristics, lung function tests, biomarkers, and other objective measurements performed at regular visits to the clinic from birth till adulthood. Analysis of biomarkers was planned in accordance to NIH guidelines 39. COPSAC serves as the de facto primary health centre for the cohorts of approximately 1100 children with regular and acute visits to the clinic. The cohorts use the COPSAC research clinic for all airway‐ and skin‐related diagnoses and treatments, avoiding the inconsistent practice in the community. This close adherence of the cohort to our research unit allows us to standardize all procedures including objective assessments, diagnostic criteria, and medical treatments.
Proposed dietary risk factors, such as vitamin D and fish oil intake during pregnancy, are likely to be subject to confounding from lifestyle factors, which can only partly be controlled for in observational studies. We therefore chose to study these hypothesized risk factors in embedded randomized controlled trials to be able to standardize these possible confounders as well as establish any causal effects. The choice of applying several randomized trials in the same cohort carries the risk of interaction between treatments that must be accounted for in the statistical analyses. The argument for doing this is that the applied interventions are environmental factors that all pregnant women are normally exposed to and often use as supplementations. Vitamin D and fish oil levels vary markedly between individuals as a result of different lifestyles in terms of sun exposure, diet, and intake of supplements, and antibiotics are commonly used as part of the treatment of asthma exacerbations 1. The randomized trials will allow us to assess potential causal effects and effect modification from these exposures that would otherwise still be present as confounding factors without standardization.
We are applying new objective methods of disease assessment to target and refine our understanding of the different endotypes. These include breathomics, metabolomics local airway immune profiling, and multiple‐breath washout, all applicable to clinical practice as non‐invasive methods.
We first applied this longitudinal clinical approach in the COPSAC2000 birth cohort. Records from the family practitioners were compared with our data from the COPSAC2000 cohort showing no missing information on atopic disorders and infections, although some under‐reporting on trauma 40. Our studies have often showed strong statistical power compared with larger study numbers presumable because of the highly specific end‐points and exposure assessments and the accurate information on time of onset.
Study compliance
The intensive regime with several hour‐long visits at the clinic every 6 months is very resource demanding for the research unit and certainly for the families often needing to take days off from work. And yet the adherence is good with approx. 80% follow‐up after 7 years in our first birth cohort, COPSAC2000. This first birth cohort only included mothers with a history of asthma that is likely to have strengthened motivation and adherence. Still, the early indications from the COPSAC2010 novel unselected birth cohort are encouraging with a very high adherence rate of 98% at age 1 year and 95% at age 2 years.
Recruitment bias
All pregnant women attending general practitioners in our recruitment area covering both urban and rural areas were targeted for an invitation to participate in the study. Regular health check during pregnancy with the general practitioner is free and recommended for all women, and the invitations were therefore without bias. Still, with a defined aim to analyse risk of asthma, eczema, and allergy, we find the expected over‐representation of these diseases compared with non‐participants. This confirms that it is unlikely that any cohort study with a disease focus will be truly unselected. The participants differed from non‐participants with respect to socioeconomic factors, including employment and income level, while there was no apparent bias with respect to other suspected risk factors such as the mode of delivery, living with pets, smoking, or alcohol intake during pregnancy.
Cohort maintenance
In an Internet‐based survey, we found a generally very high level of satisfaction with the visits to the clinic including access to acute help, level of information, appreciation from the personal at the clinic, and the sampling taken from the child (Table 3). In particular, only 9% were displeased with the repeated blood samplings from the infant.
Ethics
We are cognizant of the need to ensure the highest ethical standards when involving pregnant women and young children in research, emphasizing the autonomy and respect for the decisions of participants. The study conforms to the Declaration of Helsinki in its latest version, and the Danish Ethical Committee and the Danish Data Protection Agency have approved the projects. The safety of the randomized control study of two supplements during pregnancy has been reviewed by the Danish Medicines Agency and is monitored by the Copenhagen GCP unit. The persons involved in the study participate voluntarily and after written informed consent by both parents. The participating families are informed that the study is unlikely to benefit their child and that discoveries will only benefit future generations. The analyses of the biobank are anonymized, and the results are not provided to the families. The participants have the advantage of a consultation by a paediatrician when they have any symptoms from the airways or skin, which is expected to facilitate early diagnosis and treatment. Successful early diagnosis and effective treatment will reduce morbidity and therefore improve quality of life.
In the COPSAC2000 birth cohort study, we conducted semi‐structured qualitative interviews with parents and children to assess ethical questions pertaining to such comprehensive and invasive clinical research. It was the consistent conclusion that it is possible to conduct invasive clinical research on infants and young children in a manner that parents find ethically sound. Altruism was their primary motivation and secondly the comfort of close and easy access to paediatricians and other specialists 41, while the children themselves after 7 years of participation emphasized the friendship they had built with the COPSAC study group 42. In this novel COPSAC2010 cohort, we used Internet‐based survey tools to ask the parents anonymously about the motivation for participation (Table 3). Again altruism was the main reason given by 65% of the cohort, a particular interest in asthma, eczema, and allergy was the second most common reason given by 21% of the cohort.
Conclusion
Preventive measures against asthma, eczema, and allergy have largely failed due to our lack of understanding of the disease origins. The COPSAC studies apply a translational clinical research approach based on long‐term studies of birth cohorts with deep phenotyping and exposure assessment, genotyping, and sequencing of the microbiome. Combined with randomized controlled trials, insight into the genetic regulation of basic biological processes, and state‐of‐the‐art systems biology methods, this approach holds the promise to improve our understanding of the processes causing disease in individual patients and the interaction between heredity and environment. Identification of the environmental factors that promote or protect against future disease raises realistic hope of prevention. This may provide the basis for the development of novel diagnostic tests, identification of molecular drug targets, and the possibility of individualized treatment. This research programme will eventually have significant effect on public health, as these diseases affect approximately one‐fifth to one‐third of all families in westernized countries and may represent communality with other chronic inflammatory diseases developing later in life.
Supplementary Material
Figure S1. Diary page – translated to English.
Figure S2. Obtaining breath samples.
Figure S3. Obtaining mucosal lining fluid.
Figure S4. Handling of blood samples obtained at 18 months of age.
Figure S5. Hypopharyngeal aspiration.
Figure S6. Nasopharyngeal aspiration.
Figure S7. Asthma treatment algorithm.
Figure S8. Rhinitis classification and treatment.
Figure S9. Schematic presentation of the relationship between the randomized trials on nutritional intervention during pregnancy and the trial on azithromycin in the offspring.
Acknowledgements
The authors gratefully express their gratitude to the children and families of the COPSAC cohort studies for all their support and commitment, and we acknowledge and appreciate the unique efforts of the COPSAC research team.
COPSAC is funded by private and public research funds. The Lundbeck Foundation; the Pharmacy Foundation of 1991; Augustinus Foundation; the Danish Medical Research Council; and The Danish Pediatric Asthma Centre provided the core support for the study.
Bisgaard H., Vissing N. H., Carson C. G., Bischoff A. L., Følsgaard N. V., Kreiner‐Møller E., Chawes B. L. K., Stokholm J., Pedersen L., Bjarnadóttir E., Thysen A. H., Nilsson E., Mortensen L. J., Olsen S. F., Schjørring S., Krogfelt K. A., Lauritzen L., Brix S., Bønnelykke K., Clinical & Experimental Allergy, 2013. (43) 1384–1394
References
- 1.Bisgaard H, Szefler S. Prevalence of asthma‐like symptoms in young children. Pediatr Pulmonol 2007; 42:723–8 [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. The epidemic of allergy and asthma. Nature 1999; 402:2–4 [DOI] [PubMed] [Google Scholar]
- 3.Eder W, Ege MJ, von Mutius E. The Asthma Epidemic. N Engl J Med 2006; 355:2226–35 [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard H, Hermansen MN, Buchvald Fet al Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007; 357:1487–95 [DOI] [PubMed] [Google Scholar]
- 5.Bisgaard H, Li N, Bonnelykke Ket al Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol 2011; 128:646–52.e5 [DOI] [PubMed] [Google Scholar]
- 6.Camargo CA, Rifas‐Shiman SL, Litonjua AAet al Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007; 85:788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen SF, Østerdal ML, Salvig JDet al Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry‐based follow‐up from a randomized controlled trial. Am J Clin Nutr 2008; 88:167–75 [DOI] [PubMed] [Google Scholar]
- 8.Prescott SL. Early‐life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol 2013; 131:23–30 [DOI] [PubMed] [Google Scholar]
- 9.Bisgaard H. The Copenhagen prospective study on asthma in childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol 2004; 93:381–9 [DOI] [PubMed] [Google Scholar]
- 10.Skytt N, Bønnelykke K, Bisgaard H. To wheeze or not to wheeze: that is not the question. J Allergy Clin Immunol 2012; 130:e5. [DOI] [PubMed] [Google Scholar]
- 11.Hatz C, von Sonnenburg F, Casula D, Lattanzi M, Leroux‐Rouel G. A randomized clinical trial to identify the optimal antigen and MF59(®) adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 2012; 30; 3470–7 [DOI] [PubMed] [Google Scholar]
- 12.Bisgaard H, Pipper CB, Bønnelykke K. Endotyping early childhood asthma by quantitative symptom assessment. J Allergy Clin Immunol 2011;127:1155–64.e2 [DOI] [PubMed] [Google Scholar]
- 13.Halkjaer LB, Loland L, Buchvald FFet al Development of atopic dermatitis during the first 3 years of life: the Copenhagen prospective study on asthma in childhood cohort study in high‐risk children. Arch Dermatol 2006; 142:561–6 [DOI] [PubMed] [Google Scholar]
- 14.Giwercman C, Lerbaek A, Bisgaard H, Menné T. Classification of atopic hand eczema and the filaggrin mutations. Contact Dermatitis 2008; 59:257–60 [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H, Halkjaer LB, Hinge Ret al Risk analysis of early childhood eczema. J Allergy Clin Immunol 2009; 123:e5. [DOI] [PubMed] [Google Scholar]
- 16.Brasholt M, Baty F, Bisgaard H. Physical activity in young children is reduced with increasing bronchial responsiveness. J Allergy Clin Immunol 2010; 125:1007–12 [DOI] [PubMed] [Google Scholar]
- 17.Brasholt M, Chawes B, Kreiner‐Møller E, Vahlkvist S, Sinding M, Bisgaard H. Objective assessment of levels and patterns of physical activity in preschool children. Pediatr Res 2013; 74:333–8 [DOI] [PubMed] [Google Scholar]
- 18.Bisgaard H, Nielsen KG. Plethysmographic measurements of specific airway resistance in young children. Chest 2005; 128:355–62 [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H, Klug B. Lung function measurement in awake young children. Eur Respir J December 1995; 8:2067–75 [DOI] [PubMed] [Google Scholar]
- 20.Chow KK. Prenatal ultrasound estimation of foetal weight. Singapore Med J 1988; 29:56–9 [PubMed] [Google Scholar]
- 21.Frankenburg W, Dodds J. The Denver developmental assessment (Denver II). Denver: University of Colorado Medical School, 1990 [Google Scholar]
- 22.Wijnhoven TM, de Onis M, Onyango AWet al Assessment of gross motor development in the WHO Multicentre Growth Reference Study. Food Nutr Bull 2004; 25(1 Suppl):S37–45 [DOI] [PubMed] [Google Scholar]
- 23.Bleses D, Vach W, Slott Met al The Danish Communicative Developmental Inventories: validity and main developmental trends. J Child Lang August 2008; 35:651–69 [DOI] [PubMed] [Google Scholar]
- 24.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edn San Antonio, TX: Administration Manual. Harcourt Assessment, 2006 [Google Scholar]
- 25.Chawes BLK, Edwards MJ, Shamji Bet al A novel method for assessing unchallenged levels of mediators in nasal epithelial lining fluid. J Allergy Clin Immunol 2010; 125:1387–89.e3 [DOI] [PubMed] [Google Scholar]
- 26.Følsgaard NV, Chawes BL, Rasmussen MAet al Neonatal cytokine profile in the airway mucosal lining fluid is skewed by maternal atopy. Am J Respir Crit Care Med 2012; 185:275–80 [DOI] [PubMed] [Google Scholar]
- 27.Følsgaard NV, Schjørring S, Chawes BLet al Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med 2013; 187:589–95 [DOI] [PubMed] [Google Scholar]
- 28.1000 Genomes [Internet]. [citeret 22. September 2011]. Hentet fra: http://www.1000genomes.org/
- 29.HapMap Homepage [Internet]. [citeret 22. September 2011]. Hentet fra: http://hapmap.ncbi.nlm.nih.gov/
- 30.Hollegaard MV, Grauholm J, Nørgaard‐Pedersen B, Hougaard DM. DNA methylome profiling using neonatal dried blood spot samples: a proof‐of‐principle study. Mol Genet Metab April 2013; 108:225–31 [DOI] [PubMed] [Google Scholar]
- 31.Skogstrand K, Thorsen P, Nørgaard‐Pedersen B, Schendel DE, Sørensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem 2005; 51:1854–66 [DOI] [PubMed] [Google Scholar]
- 32.Sørensen M, Bisgaard H, Stage M, Loft S. Biomarkers of exposure to environmental tobacco smoke in infants. Biomarkers 2007; 12:38–46 [DOI] [PubMed] [Google Scholar]
- 33.Bisgaard H, Hermansen MN, Bønnelykke Ket al Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ 2010; 341:c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med 2013; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Olsen SF, Mikkelsen TB, Knudsen VKet al Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2007; 21:76–86 [DOI] [PubMed] [Google Scholar]
- 36.Lauritzen L, Halkjaer LB, Mikkelsen TBet al Fatty acid composition of human milk in atopic Danish mothers. Am J Clin Nutr 2006; 84:190–6 [DOI] [PubMed] [Google Scholar]
- 37.Armstrong JM, Metherel AH, Stark KD. Direct microwave transesterification of fingertip prick blood samples for fatty acid determinations. Lipids 2008; 43:187–96 [DOI] [PubMed] [Google Scholar]
- 38.Metherel AH, Taha AY, Izadi H, Stark KD. The application of ultrasound energy to increase lipid extraction throughput of solid matrix samples (flaxseed). Prostaglandins Leukot Essent Fatty Acids December 2009; 81:417–23 [DOI] [PubMed] [Google Scholar]
- 39.Szefler SJ, Wenzel S, Brown Ret al Asthma outcomes: Biomarkers. J Allergy Clin Immunol 2012; 129(3 Suppl.):S9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vissing NH, Jensen SM, Bisgaard H. Validity of information on atopic disease and other illness in young children reported by parents in a prospective birth cohort study. BMC Med Res Methodol 2012; 12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gammelgaard A, Knudsen LE, Bisgaard H. Perceptions of parents on the participation of their infants in clinical research. Arch Dis Child December 2006; 91:977–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gammelgaard A, Bisgaard H. Seven‐year‐old children's perceptions of participating in a comprehensive clinical birth cohort study. Clinical Ethics 2009; 4:79–84 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Diary page – translated to English.
Figure S2. Obtaining breath samples.
Figure S3. Obtaining mucosal lining fluid.
Figure S4. Handling of blood samples obtained at 18 months of age.
Figure S5. Hypopharyngeal aspiration.
Figure S6. Nasopharyngeal aspiration.
Figure S7. Asthma treatment algorithm.
Figure S8. Rhinitis classification and treatment.
Figure S9. Schematic presentation of the relationship between the randomized trials on nutritional intervention during pregnancy and the trial on azithromycin in the offspring.


