Figure 5.
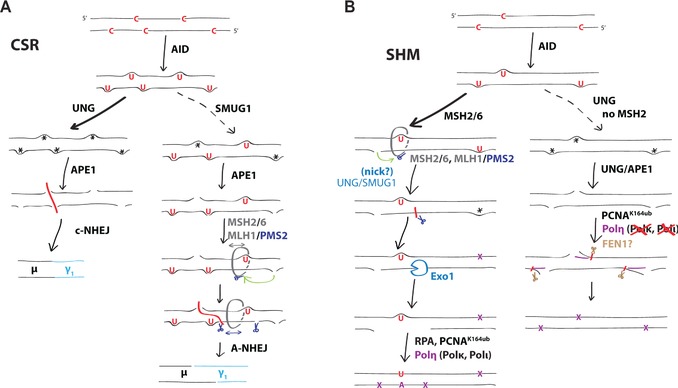
Uracil recognition pathways in antibody diversification. (A) CSR. Efficient base excision by UNG (or by highly overexpressed SMUG1) of densely spaced uracil residues in the immunoglobulin switch region repeats followed by cleavage by APE1 results in frequent double‐strands breaks that can be joined by nonhomologous end joining with minimal end processing (left path). In the absence of UNG, or in regions with lower AID hotspot density (right path), nicks are typically too far apart to allow direct formation of double‐strand breaks. Class switching thus relies on processing of U:Gs by the MMR machinery (comprising the MSH2/MSH6//MLH1/PMS2 heterotetramer) to create staggered breaks, which are resolved via microhomology‐dependent alternative end joining. Even rare nicks, mostly provided by SMUG1 in the absence of UNG, could facilitate Exo1 resection by licensing of the endonuclease activity of PMS2 in the MMR complex, and thus promote the formation of double‐strand breaks leading to class switching. (B) SHM. The contribution of uracil excision to the MHS2‐dependent phase 2 (left path) is evidenced by the lowered proportion of A:T mutations observed in the absence of UNG, which is further depressed in the absence of SMUG1. The creation of an MMR‐dependent resection patch is known to be potentiated by nicks in the region surrounding the mismatch, suggesting that uracil excision facilitates phase II by providing a source of nicks. As in the case of CSR, even isolated, distal nicks could license the PMS2 endonuclease to make further incisions and promote efficient patch resection. It is unknown, but plausible, that mutagenic MMR in the absence of uracil excision by both UNG and SMUG1 exploits other sources of nicks. Subsequent to Exo1 resection, the patch is refilled by error‐prone Y‐family polymerases, among which Polη plays the dominant role for hypermutation. In the absence of MSH2 (right path), efficient uracil excision by UNG is absolutely essential for mutagenesis at A:T pairs, and Polη cannot be substituted for by other endogenous polymerases, suggesting that they cannot (with detectable efficiency) facilitate mutagenesis across the relatively short patches of BSR.
