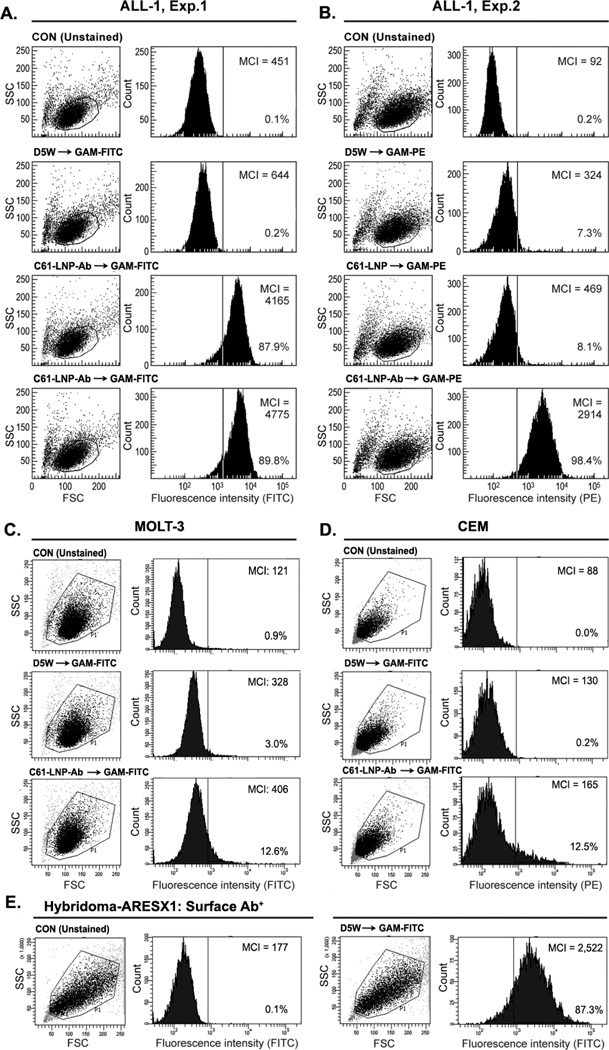
Figure 4. Immunoreactivity of Antibody-Decorated C61-LNP-Ab with B-Precursor ALL Cells.
The immunoreactivity of the C61-LNP-Ab with the CD19-receptor positive B-precursor ALL cell line ALL1 (BCR-ABL+, Pre-pre-B) was examined by standard indirect immunofluorescence and flow cytometry using FITC- (Experiment 1, Panel A) or PE-labeled goat anti-mouse (GAM) antibodies (Experiment 2, Panel B) to detect the cell-bound murine anti-CD19 MoAb moiety of the C61-LNP-Ab. Depicted are two-parameter (FSC/SSC) light scatter dot plots and single-color fluorescence histograms of ALL-1 cells stained with C61-LNP-Ab. Controls included unstained cells, cells stained with GAM alone, as well as cells stained with C61-LNP (as a non-immunoreactive LNP control). The concentration of the C61-LNP-Ab (based on C61 content) was 200 µg/mL in 3rd row in A and 400 µg/mL in 4th row in Panel A & B. The lack of a marked difference between the percent binding at the two concentrations indicates that a near complete target CD19-receptor saturation is achieved at the 200 µg/mL concentration. The concentration of C61-LNP in B was 200 µg/mL based on C61 content. The interaction of C61-LNP-Ab (400 µg/mL based on C61 content) with CD19-receptor negative human cells was also examined using two different T-lineage ALL cell lines. C61-LNP-Ab did not bind to MOLT-3 (Panel C) or CEM (Panel D) cell lines as it did to ALL-1 cells. The small percentages of MOLT3 and CEM cells (<15%) showing above background fluorescence likely reflect the ability of liposomes to interact with cellular membranes. The poor binding of C61-LNP-Ab to the CD19-receptor negative T-lineage ALL cell lines was not a false negative result caused by a technical problem related to the secondary antibody GAM-FITC, because the latter antibody exhibited strong binding to the murine hybridoma cell line ARESX1 expressing the antibody moiety of C61-LNP-Ab on its surface (panel E). MCI: Median channel of immunofluorescence. Depicted in each FACS histogram are the MCI values and the percentages of cells with above background fluorescence.