CLINICAL VIGNETTE
A 5-week-old baby boy was referred to his local immunologist to be evaluated because of abnormal T-cell receptor excision circle (TREC) newborn screening (NBS) test results. Both primary and confirmatory NBS TREC analysis showed less than 40 TRECs/µL (with a normal DNA integrity intrinsic control, the β-actin gene) on the Guthrie card sample. The patient was the second child born to a nonconsanguineous young couple who already had a 4-year-old healthy daughter. Pregnancy and delivery were unremarkable, and the baby’s weight and height at birth were 3.55 kg (50th percentile) and 50.5 cm (50th percentile), respectively.
The baby received his hepatitis B vaccine at birth and was discharged with his mother 24 hours after delivery. At his 1-month visit with the pediatrician, the baby was believed to be in good health, despite poor weight gain (0.33 kg in 1 month) but had minimal oral thrush. At that point, breast-feeding was discontinued and replaced by milk-based formula, and nystatin was prescribed. At the immunologist’s office (age 5 weeks), the physical examination still showed mild oral thrush, and his weight was essentially unchanged at 3.88 kg. No family history of immune defects or early deaths in either the maternal or paternal families was reported.
Initial laboratory evaluation ordered by the clinical immunologist yielded a complete blood count (CBC) with differential as noted in Table E1 in this article’s Online Repository at www.jacionline.org. Lymphocyte enumeration by means of flow cytometry revealed a marked T-cell and natural killer (NK) cell lymphopenia with normal numbers of circulating B cells (see Table E1). The patient has very low IgA and IgM levels, whereas IgG levels were within the normal range. These initial findings resulted in a presumptive diagnosis of T−B+NK− severe combined immunodeficiency (SCID). The patient was started on anti-infectious prophylaxis measures, and further immunologic studies were ordered.
TABLE E1.
Patient’s immunologic evaluation
| Patient (at 5 wk of age) |
Reference range (age specific) |
|
|---|---|---|
| Hemoglobin (g/dL) | 12.3 | 10.7–17.1 |
| Platelets/µL | 283,000 | 275,000–567,000 |
| WBC/µL | 8,600 | 7,200–18,000 |
| Neutrophils/µL (%) | 5,762 (67) | 2,200–6,400 (14–34) |
| Lymphocytes/µL (%) | 2,064 (24) | 3,400–7,600 (55–78) |
| CD3+ cells/µL (%) | 66 (3.6) | 2,500–5,500 (53–84) |
| CD3+/CD4+ cells/µL (%) | 33 (1.6) | 1,600–4,000 (35–64) |
| CD3+/CD8+ cells/µL (%) | 41 (2) | 650–2,450 (12–28) |
| CD19+ cells/µL (%) | 1,961 (95) | 300–2,000 (6–32) |
| CD16/CD56+ cells/µL (%) | 29 (1.4) | 170–1,100 (4–18) |
| IgG (mg/dL) | 320 | 311–549 |
| IgA (mg/dL) | <7 | 8–34 |
| IgM (mg/dL) | 9 | 19–41 |
WBC, White blood cells.
DISCUSSION
The case presented here included a very mild clinical phenotype during the first weeks of life. If not for the abnormal TREC NBS results, the diagnosis of SCID likely would not have been pursued until the baby showed clear evidence of failure to thrive, had serious infections, or both.E1 The abnormal TREC evaluation on NBS (confirmed on a repeat assay), a marker for thymic activity and lymphopoiesis, resulted in this patient being evaluated for possible severe T-cell immune deficiency, particularly SCID. The starting point for the directed evaluation of the T-cell compartment in this patient was a CBC plus differential to evaluate for lymphopenia, a finding typically seen in patients with defects of T-cell development, as well as secondary immune deficiencies associated with increased T-cell destruction (eg, HIV infection). The lymphocyte count is affected in these settings because T cells normally represent 55% to 78% of the total circulating lymphocyte population, such that a marked decrease in T-cell numbers is typically reflected by lymphopenia. In this patient the total lymphocyte count was only modestly diminished when compared with those of age-matched control subjects,E2 a reflection of the presence of circulating B cells that are at the upper end of the reference range. This mild degree of lymphopenia could also be seen with a “leaky” SCID defect that allows some T-cell development, as well as in the setting of maternal engraftment.E3 The latter would typically be detected by using karyotyping (in male patients by looking for the presence of XX maternal cells) or by using more sensitive techniques evaluating DNA short tandem repeat (STR) polymorphic markers and comparing the patient’s results with his mother’s results because one would expect the child and mother to differ (because of haploidentity) in their STR pattern. IgG levels in the patient were within the normal range, reflecting maternal IgG transplacental transport that occurred during the third trimester.
The next step in the directed evaluation of T-cell immunity involved immunophenotyping by means of flowcytometry to look at the major lymphocyte populations (ie, CD3+/CD4+ T cells, CD3+/CD8+ T cells, CD19+ B cells, and CD56/CD16+ NK cells). In this patient the results clearly pointed to a defect that interfered with both T-cell and NK cell development while leaving the circulating B-cell compartment intact (the underlying genetic defect was ultimately proved to involve the gene encoding the common γ chain, establishing the diagnosis of X-linked SCID). Additional flow cytometric studies that can be helpful in evaluating T-cell immunity include evaluation of naive versus memory T cells by using a screening approach that is based on the differential expression of CD45 isoforms, with CD45RA typically expressed by naive cells and CD45RO by memory cells. Additional markers that can further clarify naive and memory cells include CD27, CCR7, and CD62 ligand, and these can also discriminate subpopulations within the memory compartment.E4 CD31 expression is found on naive CD4+ T cells and represents another excellent cellular marker for human thymic activity.E5 It is important to recognize that these studies are not functional assays but rather identify and quantify cells based on their functional potential.
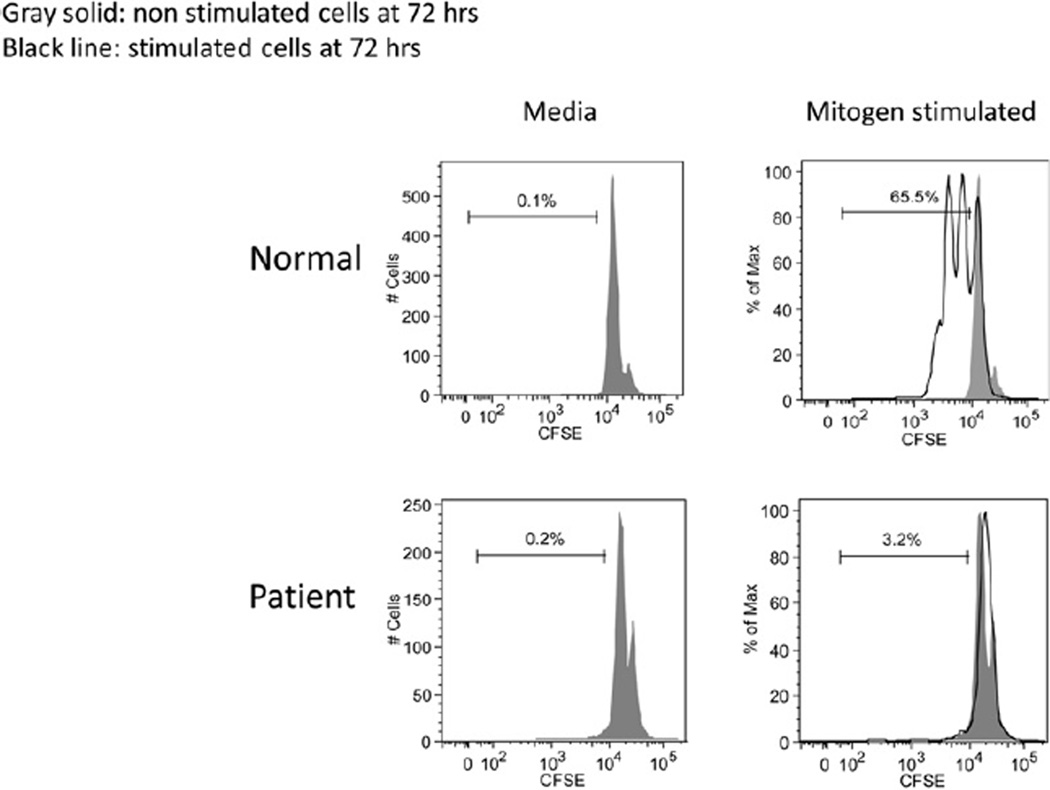
The flow studies should be accompanied by laboratory tests assessing T-cell functional immunity. This typically involves ex vivo evaluation of T-cell responses to polyclonal stimulants or mitogens (eg, PHA used in this patient, concanavalin A, and pokeweed mitogen), or anti-CD3 antibodies alone or in combination with anti-CD28 or IL-2 to determine non–antigen-specific T-cell proliferation. Generally, these assays involve a 72-hour culture of both unstimulated and stimulated cells, with the results interpreted in the context of either an established reference range or through a paired control sample run with the patient’s sample. The most common and documented method used for quantitating the mitogen response involves addition of tritiated thymidine near the end of the culture period to detect dividing cells that incorporate the labeled nucleoside into newly synthesized DNA. Quantitation involves the use of a scintillation counter to detect cellular incorporation of the radionuclide-labeled nucleoside as a reflection of cell division, with the results expressed as counts per minute (cpm). The standard way of reporting these data is simply to provide the cpm of the patient’s cells without and with a stimulant for comparison with the established reference range. An alternative approach for reporting results involves calculating a stimulation index, which is generated by dividing the stimulated cpm by the unstimulated cpm.E6 More recently, flow cytometric methods to evaluate T-cell proliferation have been described, including the use of fluorescent tracking dyes (eg, carboxyfluorescein diacetate succinimidyl ester [CFSE]) that are incorporated into the cell and after activation result in a 50% decrease in fluorescent intensity for each round of cell division (Fig E1)E7 or fluorescent nucleoside analogues (ie, 5-ethynyl-2′-deoxyuridine) that incorporate into the DNA of proliferating cells associated with a gain of fluorescent signal.E8 Additional means of evaluating the T-cell response to mitogens include flow cytometric evaluation of activation markers (eg, CD69, HLA-DR, and CD25) expressed on the responding cells at specific times after stimulation, measurement of cytokines secreted into the cell supernatant at the end of the culture period, or detection of intracellular cytokines by using flow cytometry.E9 In severe T-cell deficiency states, the T-cell response to polyclonal stimulants is typically less than 10% of the lower limit of the reference value. As noted earlier, there are “leaky” SCID mutations, as well as other T-cell deficiencies, that allow for some degree of T-cell response, although the results in this setting are most often significantly abnormal (ie, often <30% of the lower limit of the reference value). Some laboratories will use more than one dose of the mitogen or mitogens, although this is not done routinely and is generally not required to detect meaningful defects in T-cell immunity. There have been rare cases in which mitogen-induced proliferation was found to be abnormal but further evaluation with a combination of agents that directly activate T cells (phorbol 12-myristate 13-acetate and ionomycin) demonstrated that the T-cell proliferative capacity was normal but the signaling apparatus (ie, the T-cell antigen receptor [TCR]–CD3 complex) was dysfunctional.E10
FIG E1.
Flow cytometry–based lymphocyte proliferation assay using a cell-tracking dye. Cells were incubated with CFSE, a fluorescent dye that binds covalently to cytosolic proteins, and then stimulated with mitogens. CFSE dilution could be detected on the control cells after cell division/proliferation but not on the patient’s cells.
Additional ex vivo functional T-cell studies use recall antigens (eg, tetanus toxoid and Candida species antigen) to stimulate T-cell proliferation based on prior exposure to the antigen (ie, this represents an antigen-specific memory T-cell response). These assays typically require a longer culture period compared with mitogens (6–7 days vs 72 hours) and result in less overall cell proliferation, as would be expected compared with a polyclonal response. Antigen-induced T-cell proliferation might be less useful in very young children based on the lower likelihood of prior antigen exposure. Finally, proliferation to allogeneic cells can be tested based on class II MHC disparity between the patient’s responder cells and irradiated (used to prevent their proliferation) stimulator cells, an assay referred to as the 1-way mixed lymphocyte culture. This assay also is performed with a 6- to 7-day culture and quantitated by using the same methods as noted above for the response to mitogens.E6
Typically, the combination of flow cytometry and T-cell proliferation testing is sufficient to define severe defects in T-cell immunity, such as those found in patients with SCID. However, there are other assays that can be applied to answer specific questions regarding the T-cell compartment. Among these are assays to detect T-cell diversity directed at evaluating the Vβ component of the TCR. There are 2 general methods to study T-cell diversity: one is a PCR-based method, referred to as T-cell spectratyping, that evaluates diversity within each Vβ family, and the other is a flow cytometry–based method that looks at the overall distribution (proportion) of the various Vβ families, typically evaluating CD4+ and CD8+ T cells separately.E11 These assays are particularly useful in patients with T-cell defects in which circulating T cells are present that have markedly altered diversity, as seen in patients with Omenn syndrome and atypical complete DiGeorge syndrome; this type of testing is also useful in evaluating for a possible clonal T-cell disorder (malignancy).
An additional test of T-cell function that is used in a limited fashion for diagnostic purposes is T-cell cytotoxicity.E12 This is a TCR-restricted process that requires prior sensitization and uses MHC-compatible target cells that also express foreign (eg, viral) antigenic peptides. There are 2 general methods to evaluate cytotoxicity. One involves labeling the target cells with a radionuclide (eg, Cr51) and then measuring the amount of radioactivity released from lysed target cells into the supernatant after culture of the sensitized effector T-cells with the labeled target cells at various effector cell/target cell ratios. The other method uses flow cytometry to detect the expression of CD107a on the cytotoxic T cell, a process that is directly associated with the T cell–mediated cytotoxicity of the specific (MHC compatible and antigenic peptide positive) target cell (exception being perforin deficiency).E13 The Cr51 assay system is technically quite demanding and used infrequently in the standard laboratory evaluation of possible T-cell deficiency. The flow cytometric evaluation for CD107a expression on cytotoxic cells (ie, T cells and NK cells) is used commonly as a surrogate to evaluate NK cell–mediated cytotoxicity in the setting of a possible X-linked lymphoproliferative disorder or hemophagocytic lymphohistiocytosis.E13 The primary application of T cell– mediated cytotoxicity for other settings is primarily used in experimental cellular immunotherapy of cancer.
Quantitation of regulatory T (Treg) cells, which are critical for homeostasis and self-tolerance maintenance, can also be assessed by using flow cytometric studies based on intracellular forkhead box protein 3 expression in CD4+/CD25+ T cells.E14 An alternative approach involves evaluating the function of Treg cells by assessing the inhibition of T-cell activation marker expression or suppression of responder T-cell proliferation.E15
Taken together, human T-cell evaluation follows the same pragmatic and directed approach as testing other arms of the immune system: quantitative enumeration of specific cells together with evaluation of expanded characteristics of these cells (ie, CBC and differential, flow cytometry for lymphocyte population enumeration, TREC testing and other measures to characterize recent thymic emigrants, and possibly assessment of TCR diversity), as well as functional testing (ie, lymphocyte proliferation to mitogens, antigens, and/or allogeneic cells; cytokine production; T cell–mediated cytotoxicity and Treg activity) to fully characterize the defect in the specific arm of the immune system under evaluation.
THE CASE REVISITED
The mitogen-stimulated lymphocyte proliferation assay results (media [background], 3,304 cpm; PHA stimulation, 4,809 cpm; normal range of stimulated cells, 83,000–188,000; phorbol 12-myristate 13-acetate plus ionomycin stimulation, 2,982 cpm; normal range, 91,000–202,000 cpm) confirmed a severe T-cell defect before the availability of the mutation analysis. Because the low number of circulating T cells expressed CD45RO, maternal engraftment was evaluated and ruled out by using STR analysis. The decision was to proceed to hematopoietic stem cell transplantation. The patient’s sister was found to be a 10/10 HLA match, making her an ideal donor. In addition, she was evaluated and found not to carry the disease-causing IL2RG (c.C717T, p.Q235X) mutation. At age 10 weeks, the patient received an unmanipulated hematopoietic stem cell graft from his sister. Four months after receiving his nonconditioned transplant, the patient’s T cells proliferated to PHA (media, 237 cpm; PHA, 52,007 cpm), he was gaining weight (5,850 kg), and he demonstrated normal development for age. His posttransplantation course was uncomplicated, and he remained on intravenous immunoglobulin replacement therapy while awaiting evaluation of his B-cell function. This case also demonstrates the advantage of early recognition of severe T-cell defects through NBS and prompt institution of immune reconstitution before the inevitable life-threatening infectious complications seen with these disorders when left untreated.
Acknowledgments
Supported by the National Institutes of Health Intramural Research Program.
Footnotes
The full version of this article, including a review of relevant issues to be considered, can be found online at www.jacionline.org. If you wish to receive CME or MOC credit for the article, please see the instructions above.
REFERENCES
- E1.Puck JM. Neonatal screening for severe combined immune deficiency. Curr Opin Allergy Clin Immunol. 2007;7:522–527. doi: 10.1097/ACI.0b013e3282f14a2a. [DOI] [PubMed] [Google Scholar]
- E2.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- E3.Muller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98:1847–1851. doi: 10.1182/blood.v98.6.1847. [DOI] [PubMed] [Google Scholar]
- E4.Schiott A, Lindstedt M, Johansson-Lindbom B, Roggen E, Borrebaeck CA. CD27− CD4+ memory T cells define a differentiated memory population at both the functional and transcriptional levels. Immunology. 2004;113:363–370. doi: 10.1111/j.1365-2567.2004.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- E6.Stiehm ER, Ochs HD, Winkelstein JA. Immunologic disorders in infants & children. 5th ed. Philadelphia: W.B.: Saunders; 2004. [Google Scholar]
- E7.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- E8.Yu Y, Arora A, Min W, Roifman CM, Grunebaum E. EdU incorporation is an alternative non-radioactive assay to [(3)H]thymidine uptake for in vitro measurement of mice T-cell proliferations. J Immunol Methods. 2009;350:29–35. doi: 10.1016/j.jim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- E9.Maino VC, Picker LJ. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry. 1998;34:207–215. doi: 10.1002/(sici)1097-0320(19981015)34:5<207::aid-cyto1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- E10.Alarcon B, Terhorst C, Arnaiz-Villena A, Perez-Aciego P, Regueiro JR. Congenital T-cell receptor immunodeficiencies in man. Immunodefic Rev. 1990;2:1–16. [PubMed] [Google Scholar]
- E11.Toubert A, Clave E, Talvensaari K, Douay C, Charron D. New tools in assessing immune reconstitution after hematopoietic stem cell transplantation. Vox Sang. 2000;78(suppl 2):29–31. [PubMed] [Google Scholar]
- E12.Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines. 2010;9:601–616. doi: 10.1586/erv.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Bryceson YT, Pende D, Maul-Pavicic A, Gilmour KC, Ufheil H, Vraetz T, et al. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood. 2012;119:2754–2763. doi: 10.1182/blood-2011-08-374199. [DOI] [PubMed] [Google Scholar]
- E14.Graca L. New tools to identify regulatory T cells. Eur J Immunol. 2005;35:1678–1680. doi: 10.1002/eji.200526303. [DOI] [PubMed] [Google Scholar]
- E15.Canavan JB, Afzali B, Scotta C, Fazekasova H, Edozie FC, Macdonald TT, et al. A rapid diagnostic test for human regulatory T-cell function to enable regulatory T-cell therapy. Blood. 2012;119:e57–e66. doi: 10.1182/blood-2011-09-380048. [DOI] [PMC free article] [PubMed] [Google Scholar]