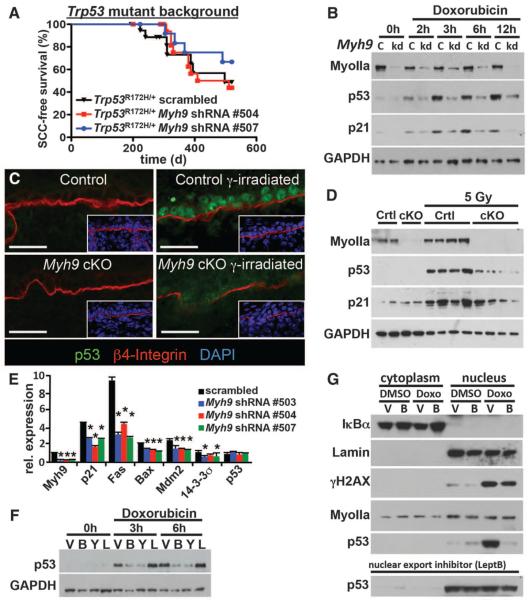
Fig. 3. A noncanonical role for myosin IIa in nuclear retention of activated p53.
(A) Tumor-free survival of conditional Trp53 mutant mice transduced with the indicated shRNA (n > 6 for each genotype; no significant survival change could be observed). (B) Myh9 knockdown (kd) but not scrambled control shRNA (c) diminishes p53 and targets p21 (CDKN2) levels in response to the DDR inducer doxorubicin. Myosin IIa and GAPDH levels are shown as controls. (C and D) Lack of nuclear p53 in Myh9-cKO versus control (Ctrl) littermate skins 6 hours after γ irradiation (5 Gy). (C) Immunofluorescence (boxed regions show DAPI-stained nuclei in blue); (D) immunoblot analysis. Myosin IIa and GAPDH levels are shown as controls. (E) Quantitative polymerase chain reaction of p53 target genes illustrates the effects of Myh9 knockdown on the p53 pathway. (F) p53 immunoblot of lysates from DDR-induced keratinocytes treated with vehicle (V), blebbistatin (B), Rho kinase inhibitor Y27632 (Y), or latrunculin B (L). GAPDH levels are shown as controls. (G) Nuclear p53 is not retained when DDR-induced Myh9 knockdown primary keratinocytes are exposed to blebbistatin (B). Lamin A/C, IκBα, and γH2AX are controls for nuclear fraction, cytoplasmic fraction, and DDR, respectively. Nuclear export inhibitor leptomycin B rescues the ability of Myh9-deficient cells to retain p53 in the nucleus.