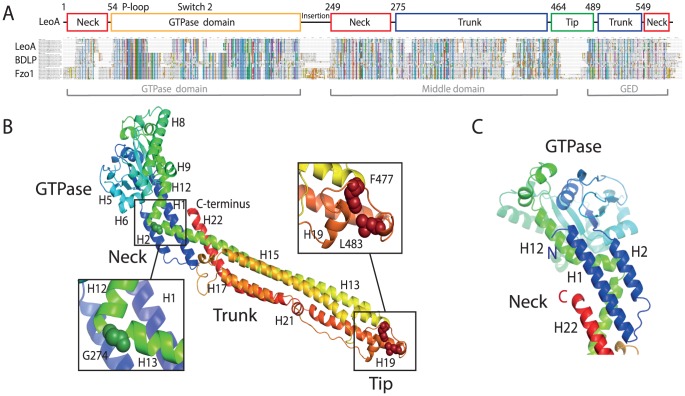
Figure 2. The crystal structure of LeoA.
A: Schematic sequence alignment showing the similarity of LeoA to Nostoc BDLP1 and eukaryotic mitofusin Fzo1. In order to make the ClustalW alignment more stable, the 10 top hits from BLAST searches, with each of the three sequences, were included for each family. Conservation stretches across all three families, with all major domains of known function and fold being conserved, leading to the conclusion that LeoA is a bona fide bacterial dynamin-like protein. B: The 2.7 Å crystal structure of LeoA from E. coli ETEC H10407 shows an elongated molecule with the conserved GTPase domain followed by the trunk and tip regions. The conformation of the trunk relative to the GTPase domain, mediated by Gly274, is novel and reveals a ‘flattened’ conformation reminiscent of that observed in the DLP human guanylate-binding protein 1 [32]. The putative paddle region at the trunk tip is dominated by hydrophobic residues in conserved positions known to be critical for lipid binding in Nostoc BDLP1 [9]. A rainbow colour scheme is used from the N (blue) to the C (red) terminus. C: Close-up of neck and GTPase domains, rotated approximately by 180° with respect to main part of panel B.