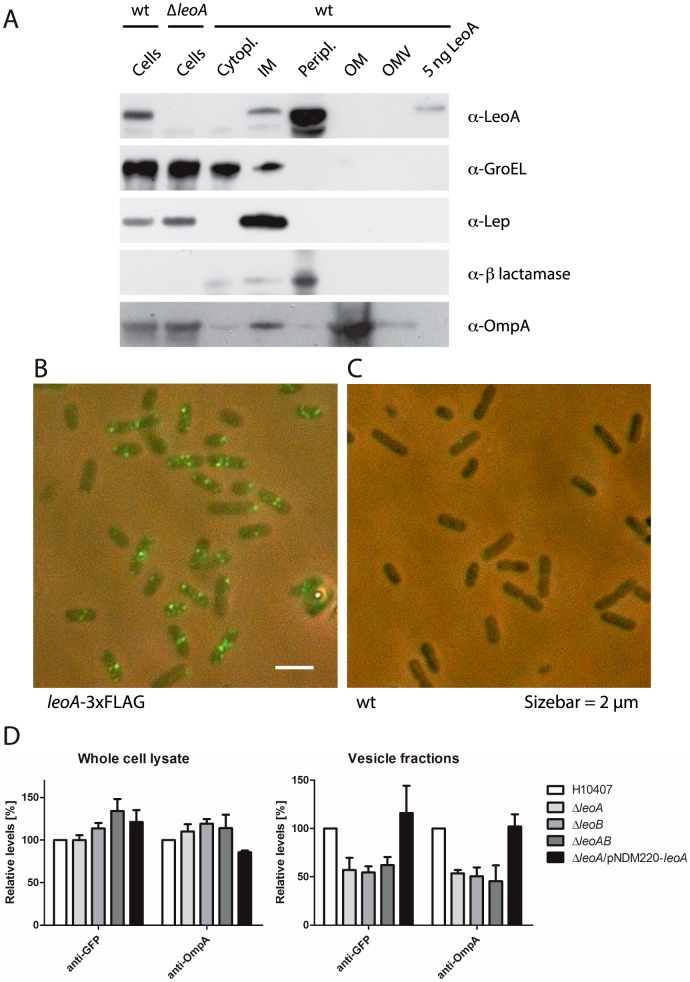
Figure 4. LeoA is localised in the periplasm and the leoAB genes enhance vesicle-based protein export.
A: Using a polyclonal LeoA antibody, the sub-cellular localisation of LeoA in WT E. coli ETEC H10407 was investigated. The protein at endogenous expression levels is easily detected and this signal disappears in a leoA KO strain, demonstrating specificity. A classical fractionation experiment with sub-cellular marker proteins clearly shows LeoA to be localised in the periplasm and possibly in the inner membrane. Figure S5 in File S1, top shows the same experiment with a 3xFLAG-tagged version of LeoA. B: Immunofluorescence using the leoA-3xFLAG fusion strain shows a punctate pattern, which is specific to the presence of the fusion (C, right). See Figure S2 in File S1 for quantification of preferential polar and midcell localisation. D: Quantified Western blots demonstrating the influence of Leo proteins on protein secretion into the culture supernatant, presumably via vesicles. Both OmpA and a twin arginine-exported GFP reporter construct tend to accumulate in H10407ΔleoAB whole-cell lysates (left). GroEL served as an internal loading control (Figure S6 in File S1 shows the original blots and quantification data from two biological replicates). Right: conversely, OmpA and Tat-GFP levels are reduced by about 50% in culture supernatants (vesicle fractions) from the H10407ΔleoAB mutant strain. Supplying extra LeoA protein from a plasmid reverses the effect of the leoA deletion (last column).