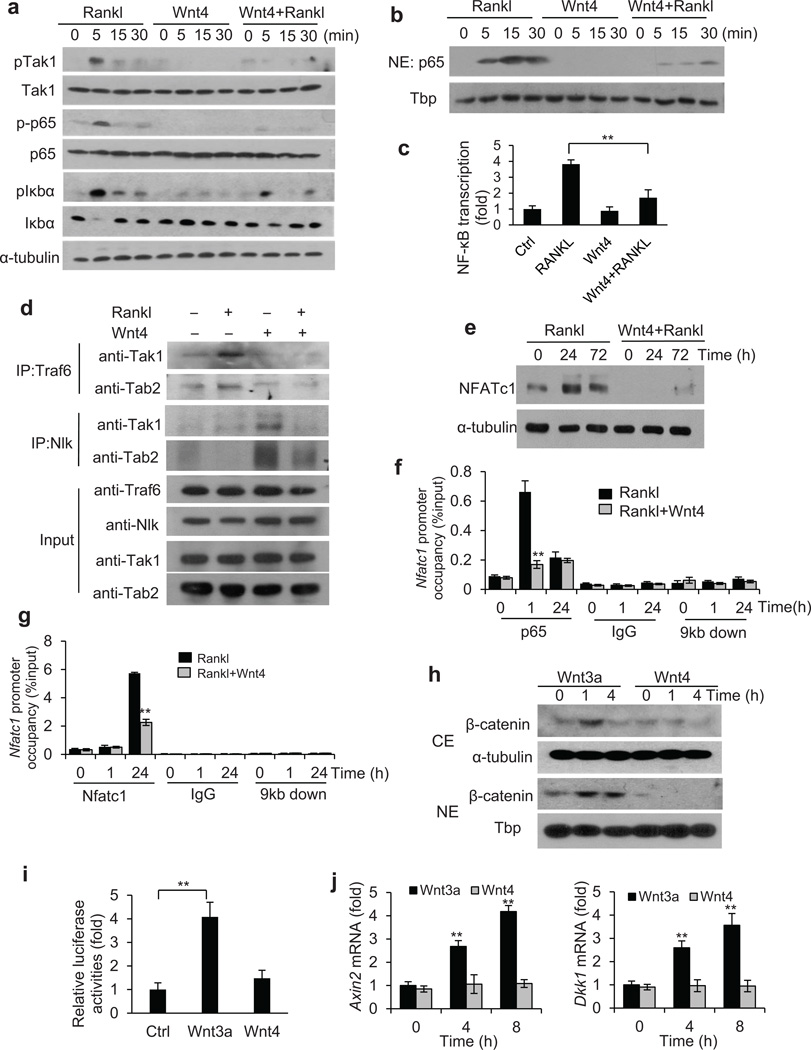
Figure 5.
Wnt4 inhibits NF-κB by interfering with TAK1-TRAF6 binding. (a) Immunoblots showing the phosphorylation of Tak1, p65 and Iκbα in bone marrow macrophages after treatment of Rankl, rWnt4 and rWnt4 with Rankl. (b) Immunoblots showing p65 and Tata-binding protein (Tbp) in nuclear extracts of bone marrow macrophages treated with Rankl, rWnt4 and rWnt4 with Rankl. (c) Relative NF-κB-dependent luciferase reporter activities in bone marrow macrophages after treatment of Rankl, rWnt4 and rWnt4 with Rankl. (d) Immunoblots showing the Traf6-Tak1-Tab2 complex formation induced by Rankl in bone marrow macrophages. (e) Immunoblots showing the induction of Nfatc1 in bone marrow macrophages after treatment of Rankl, and rWnt4 with Rankl. (f) ChIP assays of the recruitment of p65 to the Nfatc1 promoter induced by Rankl. Anti-IgG and primers designed at 9 kb downstream of transcription start site (TSS) were used as negative control. (g) ChIP assays of Nfatc1 binding to the Nfatc1 promoter. (h) Immunoblots of β-catenin in cytosolic extract (CE) and nuclear extract (NE) of bone marrow macrophages treated with Wnt3a and Wnt4. (i) Relative Topflash luciferase activities in bone marrow macrophages treated with Wnt3a or Wnt4. (j) Real-time RT-PCR of Axin2 and Dkk1 in bone marrow macrophages treated with Wnt3a or Wnt4. n = 3; * P < 0.05; ** P < 0.01; unpaired two-tailed t-test.